14
DecemberSFDA workshop on quality variation in pharmaceutical products (Requirements and conditions)
Main building - Training and Conference Center
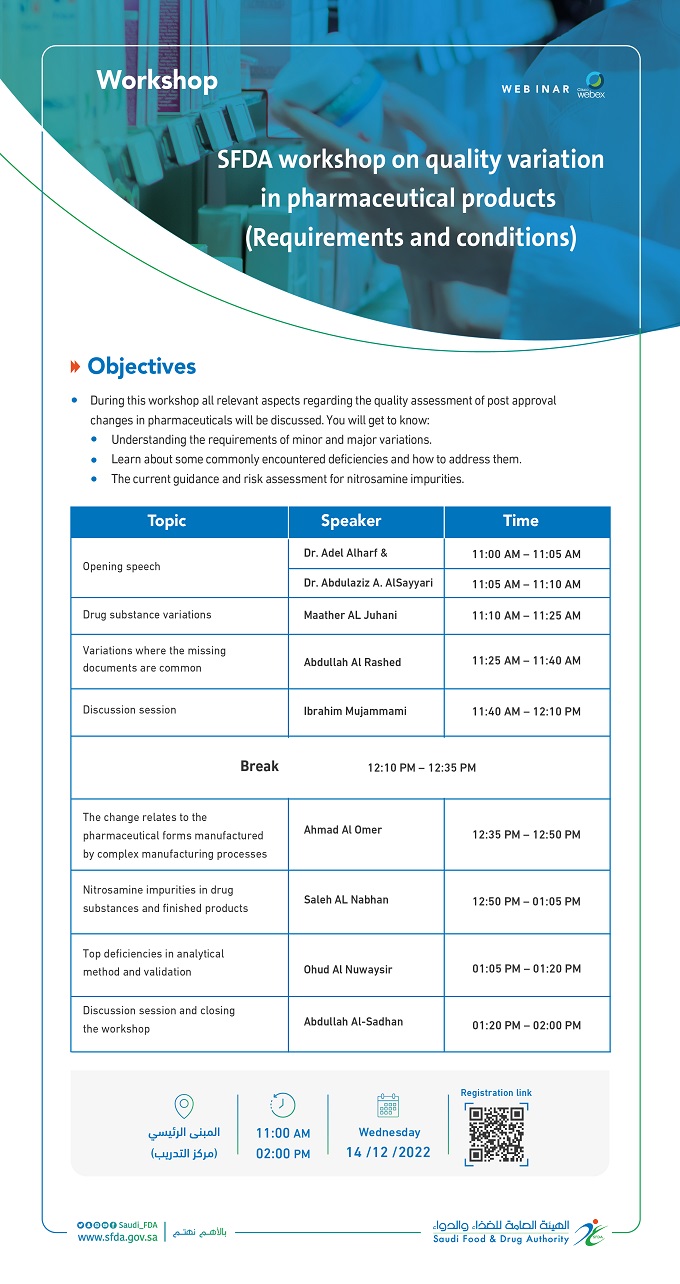
» Objectives
During this workshop all relevant aspects regarding the quality assessment of post approval changes in pharmaceuticals will be discussed. You will get to know:
- Understanding the requirements of minor and major variations.
- Learn about some commonly encountered deficiencies and how to address them.
- The current guidance and risk assessment for nitrosamine impurities.
| From Date |
|
| To Date |
|
| Workshop Type |
Public
|
| Presenting Language |
English
|
The workshop is over





