SFDA Captures 45 Pharmacies Selling Violating Products
2015-11-11
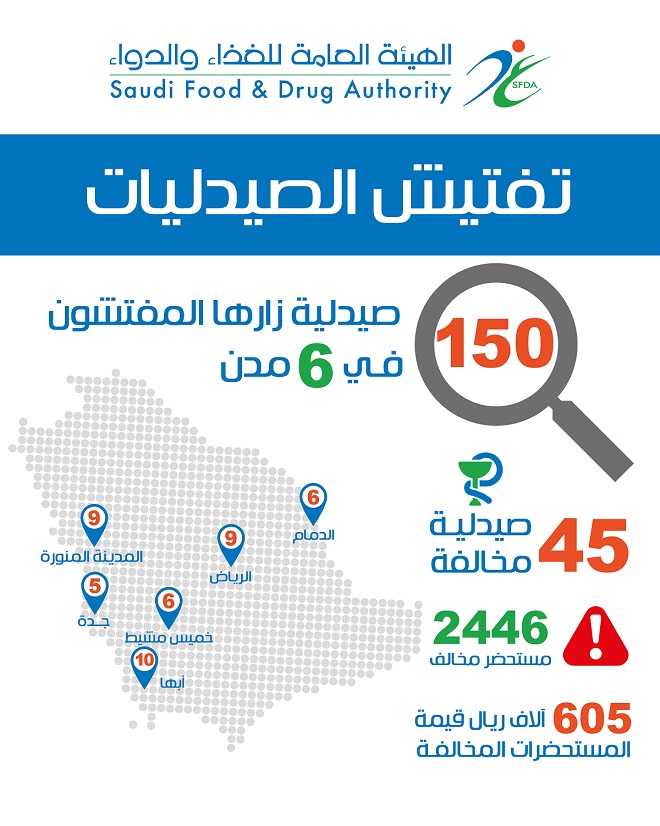
The inspection tours conducted by SFDA inspectors to 150 pharmacies kingdom wide resulted in capturing 2446 violating products in 45 pharmacies during only one month.
This campaign came as part of SFDA Control plans and surveillance and Inspection Programs to ensure compliance and validity of the products marketed in pharmacies to the regulations
Among 20 pharmacies visited by SFDA inspectors in Riyadh, 1181 violating products were captured in 9 pharmacies. Among 20 pharmacies visited in Jeddah only 5 violating pharmacies were detected, with 157 violating products..
In Dammam, SFDA inspectors found 6 violating pharmacies, among 20 pharmacies covered by inspection and detected 141 violating products. In Madinah, 9 violating pharmacies were detected, among 30 pharmacies visited by the inspectors, with 494 violating products. In Khamis Mushayt, the inspection covered 30 pharmacies, wherein 183 violating products were detected in 6 pharmacies> Moreover, 30 pharmacies were inspected in Abha wherein the inspectors captured 293 violating products in 10 pharmacies.
SFDA is currently proceeding with the legal actions against violators, according to regulations together with destruction of the violating quantities that worth over SR 605 Thousand.
SFDA inspectors visited 60 pharmacies in Riyadh, Jeddah and Dammam during Eid Al Adha holidays and captured 1479 violating products.
Also, during the month of Ramadan, SFDA inspectors captured more than 50 thousand packs of violating pharmaceutical products in several pharmacies and warehouses and found more than 20 thousand packs of pharmaceutical products in poor storing conditions at an apartment belonging to a medical center in Riyadh.
SFDA stressed that it will continue with its surveillance tours in the different regions of the Kingdom in order to ensure safety, quality and efficacy of drugs and that no cheating or violations exist in selling and marketing of the products within its control. SFDA called on consumers to report any drug side effects to the National Pharmacovigilence Center (NPC) at Telephone No. 011203222 Extensions: 2356, 5721, 2340, 2354 or Fax No. 0112057662 or E-mail :