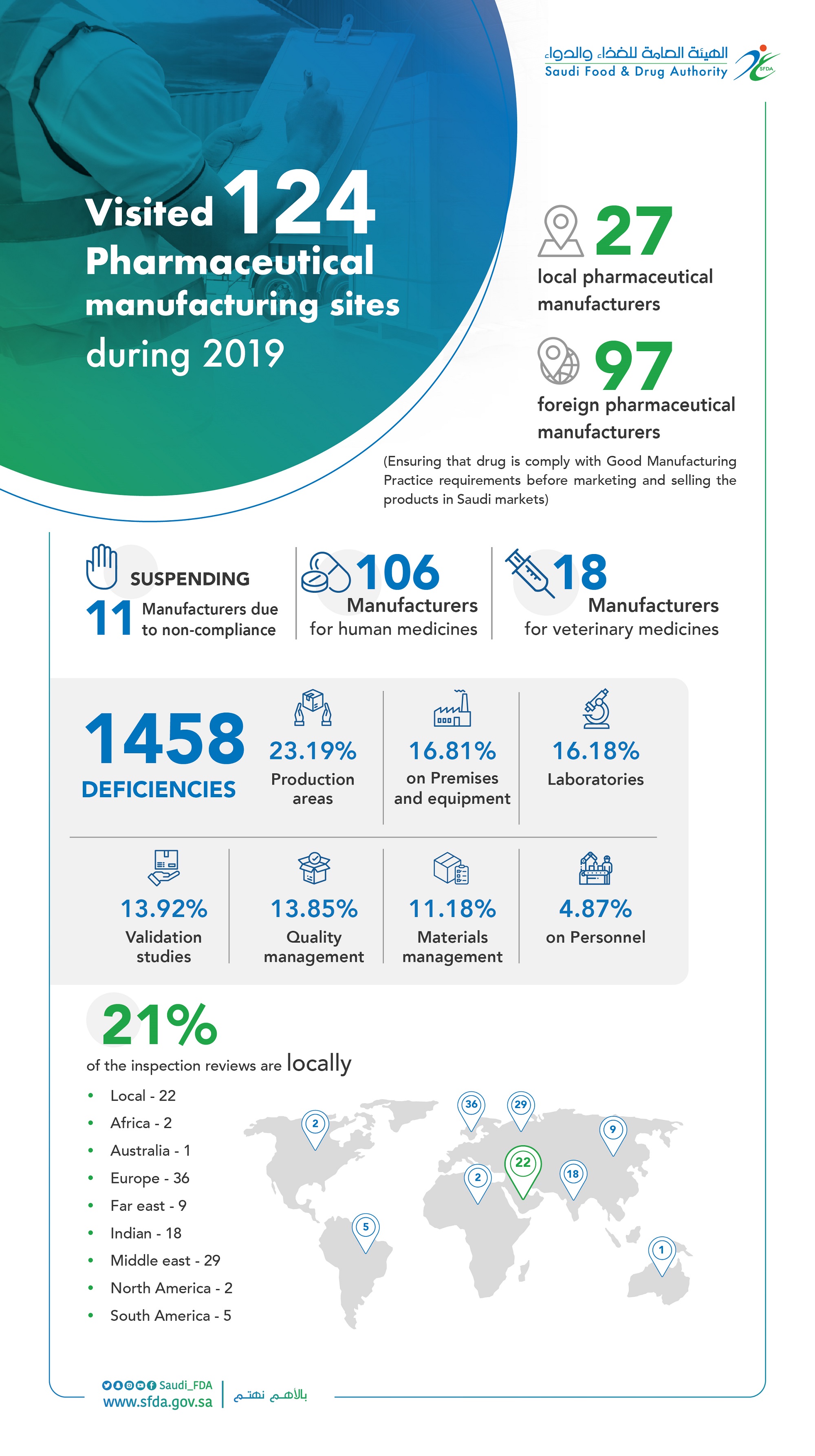
The “SFDA” Inspects 124 Domestic and Foreign Pharmaceutical Manufacturers and Suspends the Registration of 11 of them in 2019.
2020-08-17
The SFDA inspectors visited 124 domestic and foreign pharmaceutical manufacturers during 2019.
It aims to ensure compliance with Good Manufacturing Practice (GMP) requirements before starting market and sell the products in the local markets, to guarantee the safety of medicine in the Saudi Arabia.
The inspection tours conducted by SFDA inspectors resulted in suspending 11 manufacturers due to non-compliance with Good Manufacturing Practice, and seizing 1458 violations included production areas at a rate of 338 notes, 245 on buildings and production machines, 236 on laboratories, 203 on verifications, 202 on quality management, 163 on materials management and 71 notes for employees in manufacturers.
The SFDA inspectors covered 106 manufacturers for human medicines and 18 for veterinary medicines.
The total number of inspection tours have reached 22 manufacturers, representing 21% of the total rate of visits and102 foreign manufacturers, as follows: 36 manufacturers in Europe,29 manufacturers in Middle East, 18 manufacturers in India, 9 manufacturers in Far East, 5 manufacturers in South America, 2 manufacturers in each of North America and Africa and manufacturer in Australia.