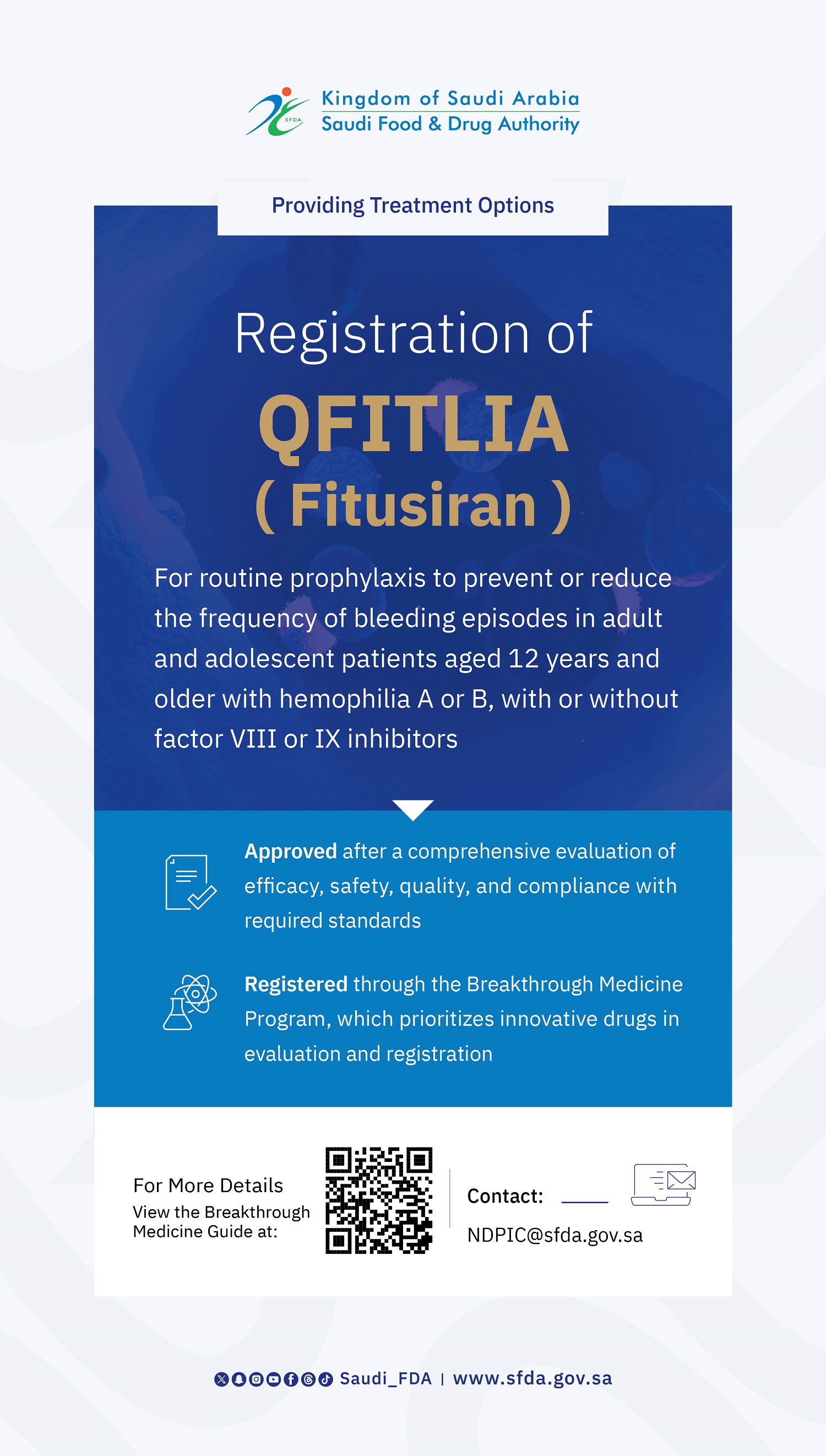
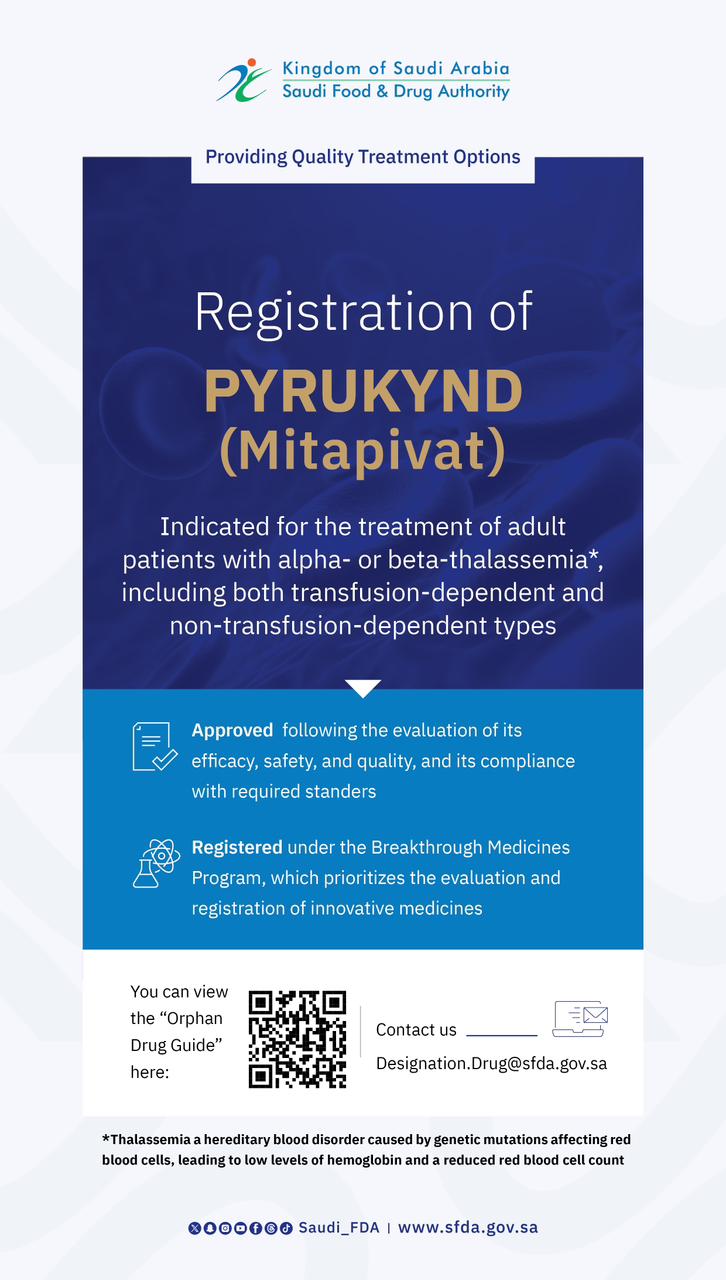
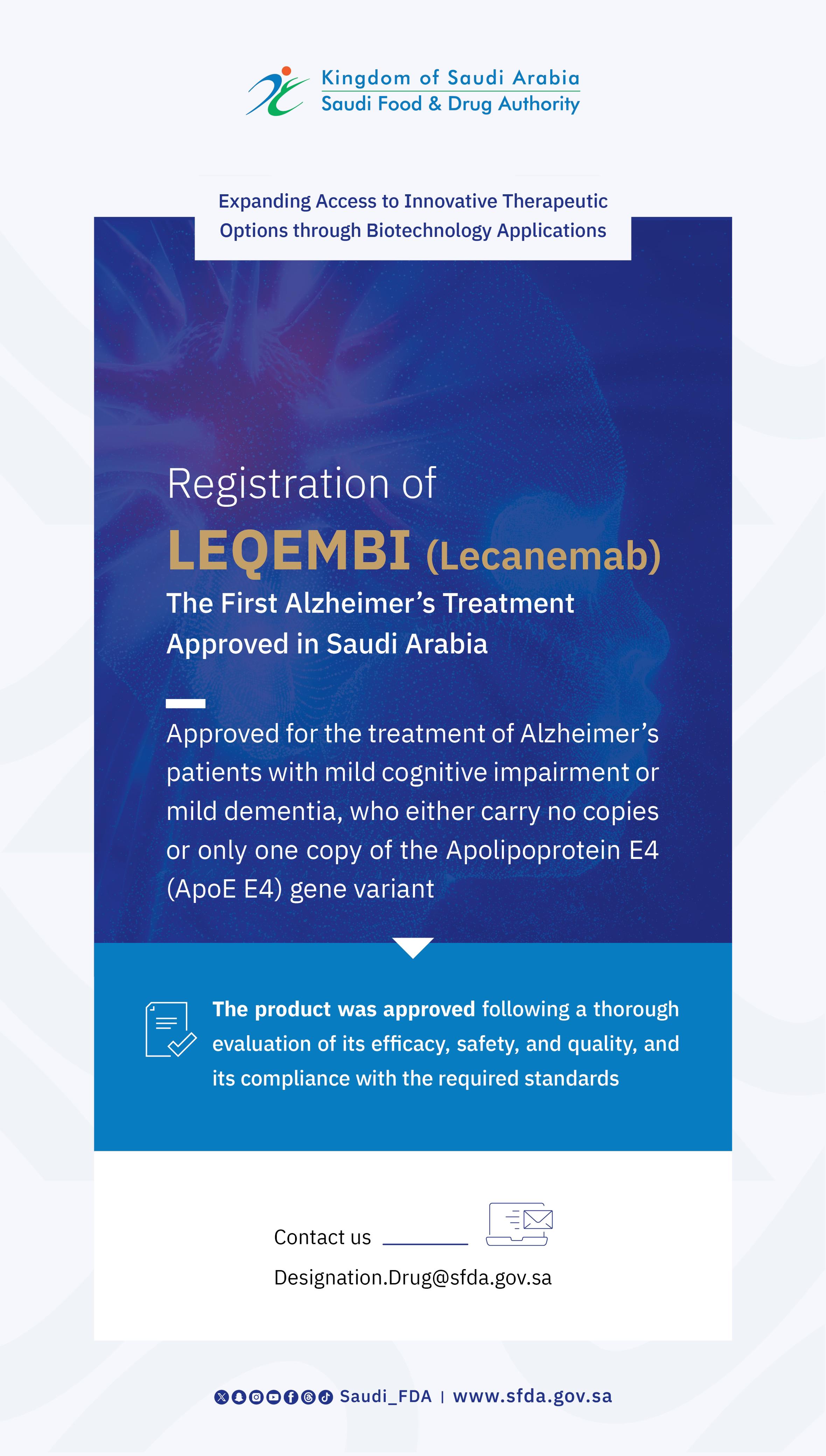
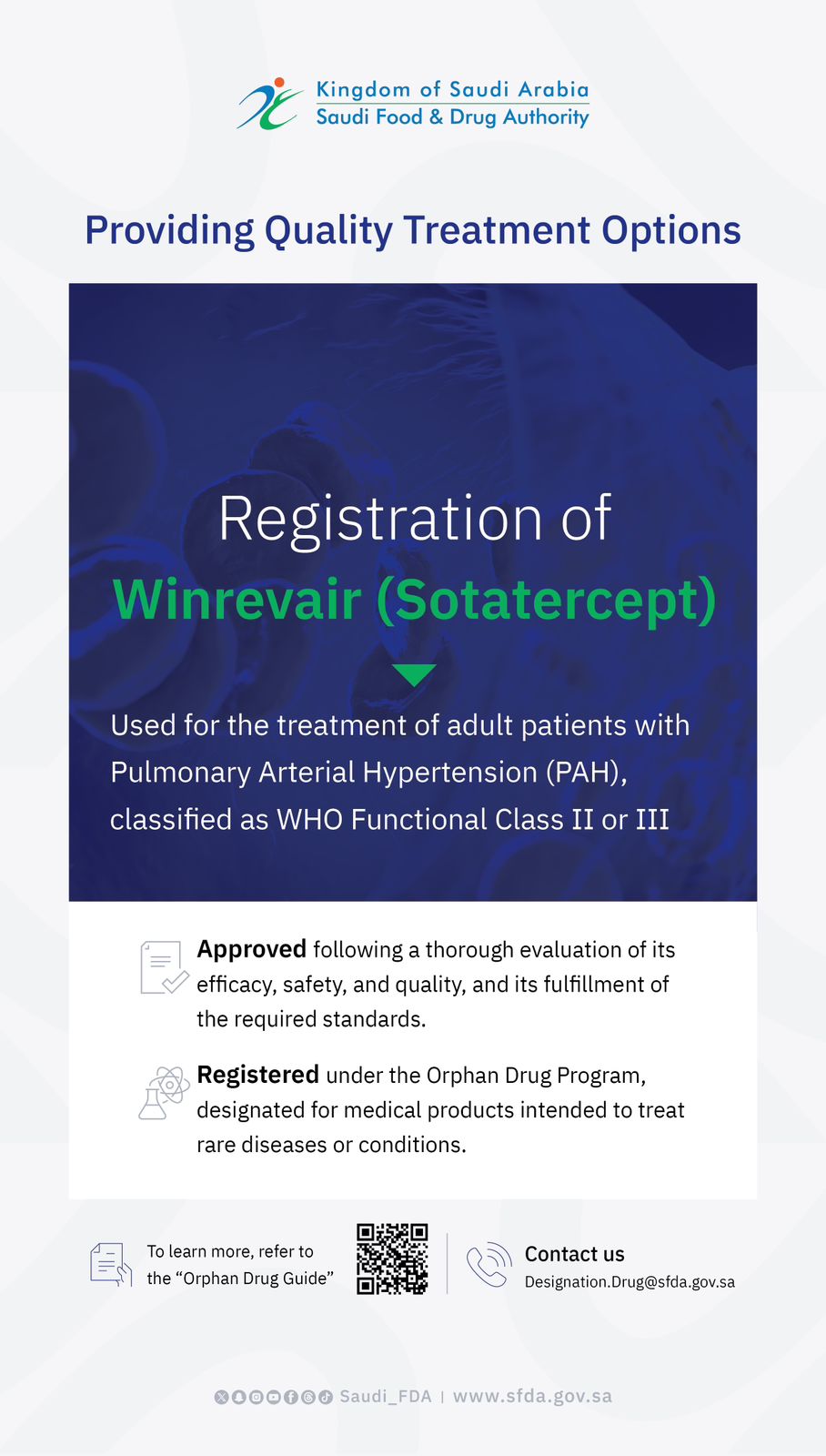
SFDA Approves Registration of “Anktiva” for the Treatment of Bladder and Lung Cancer
2026-01-16
The Saudi Food and Drug Authority (SFDA) has granted conditional approval for Anktiva (nogapendekin alfa inbakicept) in combination with immunotherapy for adult patients with metastatic non-small cell lung cancer (NSCLC) whose disease has progressed after standard of care. The SFDA is the first regulatory authority globally to grant conditional approval for this medication for the treatment of NSCLC.
Additionally, the SFDA approved the use of Anktiva in combination with Bacillus Calmette–Guérin (BCG) for adult patients with high-risk, BCG-unresponsive non-muscle-invasive bladder cancer (NMIBC) with carcinoma in situ (CIS).
Innovative mechanism of action through interleukin-15 (IL-15) receptor agonist
Anktiva has a novel mechanism by binding to IL-15 receptor resulting in proliferation and activation of natural killer (NK) cells, CD4+, CD8+, and memory T cells, without the proliferation of immuno-suppressive Treg cells.
For NSCLC patients, Anktiva is administered via subcutaneous injection; for NMIBC patients, it is delivered via intravesical instillation directly into the bladder.
Positive Results Demonstrated in Clinical Studies
The approval of Anktiva is based on a comprehensive evaluation of all available evidence—including efficacy, safety, and quality—in accordance with regulatory requirements. For NSCLC, results from a single-arm clinical trial in patients who had previously not responded to one or more therapies, including immune checkpoint inhibitors, demonstrated a potential improvement in survival. Based on these findings, the SFDA granted conditional approval for this indication. A confirmatory trial to demonstrate long-term clinical benefit is required to maintain this approval status.
For NMIBC, clinical trial results sowed a complete response rate of 62%. The approval for this indication was based on the complete response rate as the primary endpoint. Collectively, these results led the SFDA to consider Anktiva as a new treatment option for patients with limited therapeutic alternatives, contributing to improved disease and survival outcomes.
Common Side Effects
The SFDA reported that the most common adverse events in bladder cancer clinical trials included elevated creatinine, painful or difficult urination, blood in the urine, and urinary urgency or frequency. Other reported side effects included urinary tract infections, increased potassium, muscle and bone pain, chills, and fever.
In lung cancer trials, the most common adverse events included injection-site reactions such as redness, pain, or itching, as well as chills, fatigue, fever, nausea, influenza-like symptoms, and loss of appetite.
Sustained Efforts to Achieve National Objectives
This approval reflects the SFDA’s continued commitment to innovation and expanding access to advanced treatment options, thereby enhancing the quality of healthcare in alignment with the goals of the Health Sector Transformation Program, one of the key initiatives of Saudi Vision 2030.