Post-Market Evaluation for the Safety of Surgical Staplers for Internal Use
Post-Market Evaluation for the Safety of Surgical Staplers for Internal Use
Surgical staplers, mechanical suturing, are mainly used to wound closure, organ or tissue resection, organ or tissue transection, and anastomoses in efficient and sterile manner [1]. Surgical staplers are generally made out of strong metals like stainless steel and titanium [2]. Strong metals were found to deliver staples with fewer rates of associated complaints [2]. The working principle of surgical staplers is basically inserting disposable cartridges to seal wounds. Figure 1 demonstrates the basic types of surgical staplers. The staplers could be used internally or externally in accordance with the surgical needs

However, there are some concerns regarding the safety of surgical staplers for internal use in an international level. A number of 21 reports of adverse events were received in the National Center of Medical Devices Reporting (NCMDR) database, which raised questions about the current practices at healthcare facilities in Saudi Arabia.
|
Table 1: Adverse events according to the survey responses |
|||
|
Complication |
Occurrence |
Reported to SFDA |
Reported to the manufacturer |
|
Stapler Jam |
1 |
No |
No |
|
Malformed staples |
0 |
NA |
NA |
|
Stapler cot cutting |
0 |
NA |
NA |
|
Misfire |
0 |
NA |
NA |
|
No staple formation |
0 |
NA |
NA |
|
Partially stapling |
0 |
NA |
NA |
|
Locked on the tissue |
1 |
No |
No |
Awareness Level of Potential Risks.
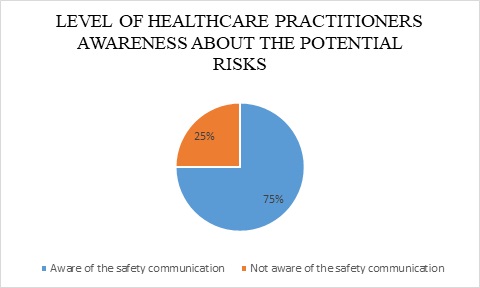
Multiple elements need to be measured to reveal the comprehension of healthcare practitioner’s community regarding the safety of certain medical devices, which are the awareness of potential risks of the device and the means to mitigate such threats. Nevertheless, figure 2, and as revealed by the Saudi users responses, demonstrates that 75% of Saudi users are aware of the published safety communication that highlighted potential risks of surgical staplers. The SFDA safety communication was published in reflection to the risk analysis report, to point out the devices’ potential risks (Safety communication).
Part III: Conclusion
The previous results and analysis provide indications of the actual practices of utilizing surgical staplers for internal use within the Saudi healthcare facilities. Such indications highlight primarily the following aspects:
I. Lack of adverse events reporting in patients with stapler jam and stapler locked on tissue, as these adverse events are not available at the NCMDR database.
II. Lack of the device user’s awareness of the potential risks of the device, as indicated in the SFDA published safety communications.
After analyzing the responses of the device users in the Saudi market, the following set of actions are recommended:
i. Provide a plan to increase the awareness of the device users regarding the risks associated with the device and the proper practices to avoid adverse events.
ii. This plan is to be reviewed by the SFDA staff, and whenever approved, the manufacturer is committed to apply it in all sites that use the device in question.
iii. To encourage healthcare practitioners to report any complaints or adverse events they encounter with surgical staplers through the National Center for Medical Devices Reporting (NCMDR) system: https://ncmdr.sfda.gov.sa, the Saudi Vigilance System https://ade.sfda.gov.sa, or the SFDA call center: 19999
Thanks to the post-market clinical evaluation team for their supports in conducting this work. For further information or inquiries related to this study, you may contact us at: cia.md@sfda.gov.sa
[1] Alicia D. Gaidry, Laurier Tremblay, Don Nakayama, Romeo C. Ignacio, Jr., "The History of Surgical Staplers: A Combination of Hungarian, Russian, and American Innovation," Sage Journals, vol. 85, no. 6, pp. 563-566, 2019.
[2] Terry Turner, "Surgical Staplers," drugwatch, [Online]. Available: https://www.drugwatch.com/surgical-staplers/.
[3] "History and Principle of Surgical Stapling," http://www.oc.lm.ehu.es.
[4] Saudi Food and Drug Authority. (2023). Requirements for Post-Market Surveillance of Medical Devices (MDS-REQ 11). Retrieved from https://sfda.gov.sa/sites/default/files/2023-05/MDS_REQ%2011_V2_En.pdf


