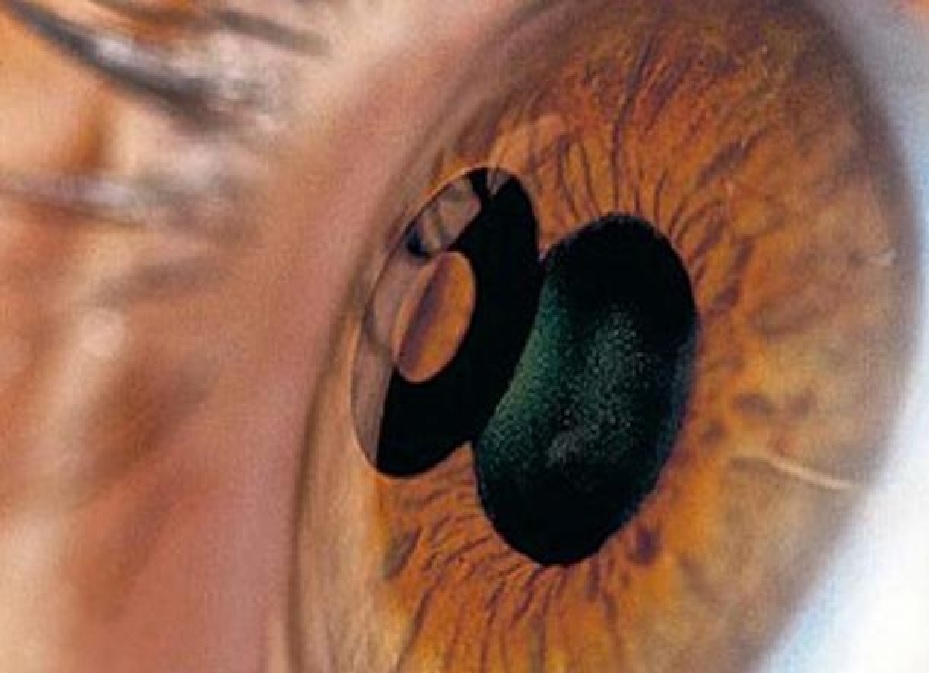
SFDA Recommended Protective Actions to Patients of Implanted KAMPRA™ Lense
2015-06-04
SFDA recommended protective procedures to patients of implanted KAMPRA™ Lenses in case they undergo laser operations , or Femtosecond Laser, or Retinal Laser Photocoagulation operations for removal of Glaucoma .
SFDA clarified that AcuFocus ™ Inc. Company, the manufacturer of KAMPRA™ Lenses, has updated their circuited notice dated 27 Sha’ban 1435H, to reflect the additional protective actions for patients of implanted KAMPRA™ Lenses applications .
SFDA advised patients undergoing laser operations for removal of Glaucoma to first remove implanted KAMPRA™ Lenses before using laser operations for removal of Glaucoma. The manufacturer confirmed that they did not receive any reports about problems of using laser operations applications, but have recommended this protective action.
The manufacturer advised people who will undergo Femtosecond Laser operations not to use Femtosecond Laser operations Not to use Femtosecond Laser applications for treatment of patients with implanted such lenses, as the manufacturer received a report about a patient with implanted type of these lenses whose lenses dimensions shirked from their original size after several months of using Femtosecond Laser with this patient. Evidence of surface wholes and change of focal color was found.
As for Retinal Laser Photocoagulation operations, the manufacturer recommended removal of KAMPRA™ Lenses before undergoing any other retina operations as the manufacturer received a report about a patient who was affected by serious thermal damage of the lenses and a secondary damage of the retina after six months of using this type of laser in the presence of the lenses.
SFDA called on patients to report medical devices and products problems to the national center for medical devices reports through the following link: http://ncmdr.sfda.gov.sa