SFDA Strengthens Global Standing Through Regulation of Medical Device Clinical Studies
2025-07-28
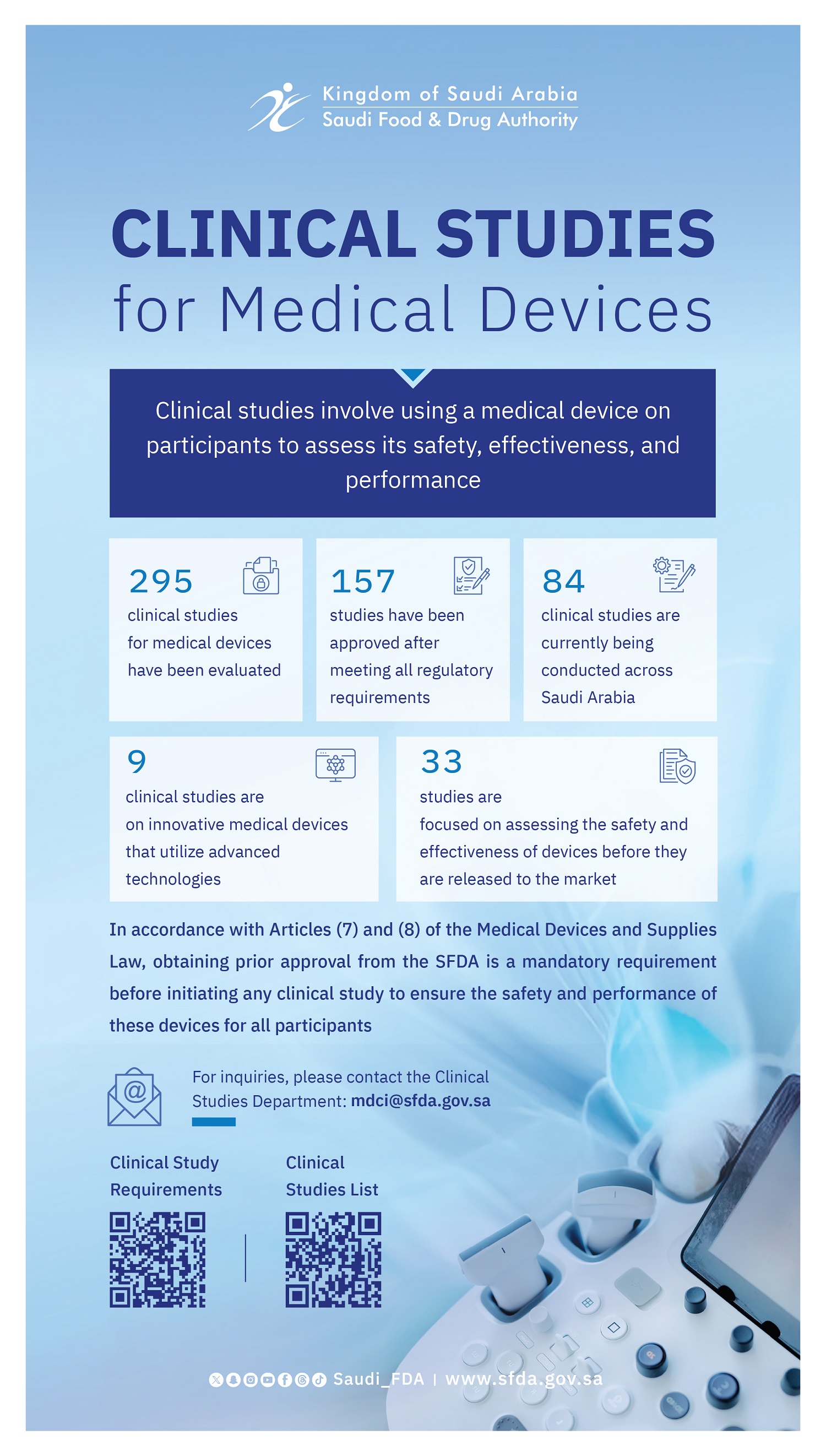
The Saudi Food and Drug Authority (SFDA) continues its efforts to regulate clinical studies for medical devices, a fundamental requirement for evaluating their safety and performance. This initiative is part of the SFDA’s regulatory and supervisory role and its commitment to strengthening its position among the world's leading regulatory authorities.
The SFDA announced that it has evaluated a total of 295 clinical studies for medical devices, approving 157 after they fulfilled all regulatory requirements. Currently, 84 of these clinical studies are actively being conducted across various regions of Saudi Arabia.
Of these approved studies, 33 are focused on assessing the safety and effectiveness of devices before they are released to the market. This includes 9 clinical studies on innovative medical devices that utilize advanced technologies. These efforts are a key part of the SFDA's strategy to support innovation and stimulate research and development in this vital sector.
The SFDA emphasizes that obtaining prior approval is a mandatory requirement before initiating any clinical study on medical devices. This is in accordance with Articles (7) and (8) of the Medical Devices and Supplies Law, which ensures the safety and performance of these devices for all participants.
For more information on clinical studies for medical devices, please contact the Clinical Studies Department at (mdci@sfda.gov.sa).
A list of all registered clinical studies can be accessed at (https://www.sfda.gov.sa/en/clinical_trials_list).