An analysis for the safe-use of CGM that relate to the training requirements
An analysis for the safe-use of CGM that relate to the training requirements
Continuous Glucose Monitoring )CGM( is a new technology and convenient technique for monitoring glucose level, that provides real-time glucose readings which can give a complete picture of variations in glucose levels. In addition, it is easy to use that sensor remains in place for many days, and it is pre-calibrated systems which remove the need for daily fingerstick. Thus, usage of CGM is an effective way to monitor glucose levels, that increases understanding the changing of blood glucose throughout the day. In order to use CGM adequately, patients must have a sufficient training and professional coaching prior to use which may lead to avoid any hazardous or potential risk may occur. The purpose of this study is to evaluate the compliance of the safe-use of CGM that relate to the training requirements.
Part I: Methodology
In this evaluation, two methodological approaches were followed. The first method is to analyze data of the previous study titled “Diabetic patients experience of using glucose monitoring devices in Saudi Arabia: A cross-sectional study”. The second method is to review adverse events through the database of the NCMDR for CGM.
Part II: Results
1- An analysis of the data of the previous study entitled “Diabetic patients experience of using glucose monitoring devices in Saudi Arabia: A cross-sectional study”:
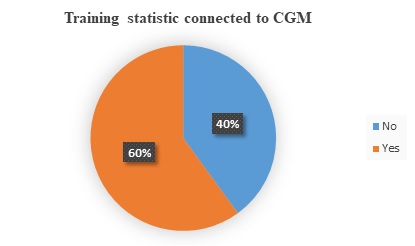
Glucose monitoring devices are widely used in Saudi Arabia. For this aim, a cross sectional study has been reported by SFDA on diabetic patients who use glucose monitoring devices to find the practices, attitudes and side effects related to these devices (1). This study was intended to discover many different brands of glucose monitoring devices and CGM devices. One of the main parts of this study was about receiving sufficient training for patients on how to use glucose monitoring devices. Figure1 demonstrates that 40% of patients didn’t receive sufficient training when using CGM, and they used it based on their knowledge without professional guidance by their healthcare providers.
2- NCMDR data of CGM adverse events:
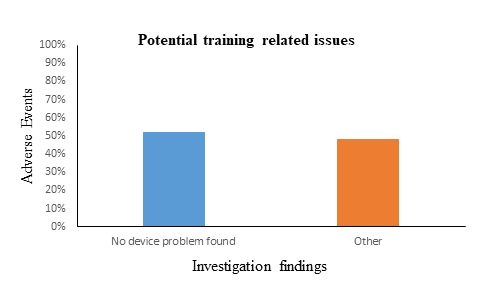
This section aims to evaluate the adverse events that reported to the NCMDR for the period of 2019 to 2021 (2). As illustrated in figure 2, the adverse events are classified into two sections based on the investigation findings. About 52% of the investigation findings of adverse events were indicated under the category “no device problem found”. According to IMDRF, this term means that the device either functioned as intended or a problem was not found. In other words, the device works properly, and the issue is most likely arising from the misuse of the device. The reason behind majority of the adverse events is cause traced to user that resulting from lacking of training. To highlight the importance of the training, a case was directed to get training following the reporting which resulting in resolving the issue. As a result, comprehensive training on using CGM devices is very crucial for patients that will lead to decrease the risk and the adverse events significantly.
Part III: Conclusion
To sum up, the analyzed results indicate that the compliance rate to the safe-use requirements for training of using CGM devises is unsatisfied. Therefore, patients must be trained adequately by healthcare provider to be able to make optimal usage of the devices safely and effectively, and to reduce the potential risk may occur.
Based on the study findings, the following actions are recommended:
• Healthcare providers should follow the indications for use (IFU) and make sure that the system is intended for single patient use and requires a prescription.
• Healthcare providers should ensure to provide patients with the necessary trainings on how to use the device properly.
• Awareness materials about the Continuous Glucose Monitoring devices.
Thanks to the post-market clinical evaluation team for their supports in conducting this work.
For further information or inquiries related to this study, you may contact us at: cia.md@sfda.gov.sa
[1] T. Aldhirgham, O. Albalawi, . J. Masoudi, B. Aloufi, M. Alhayan and . M. Majrashi, "Diabetic patients experience of using glucose monitoring devices in Saudi Arabia: A cross-sectional study," SFDA, 2021.
[2] SFDA, "National Centre for Medical Devices Reporting," 2019. [Online].


