
-
About SFDA
About SFDA
SFDA in vision 2030
Authority Strategy
Career and Life
- Information Lists
-
Areas
- Consumer Corner
- Media Centre
- Eservices
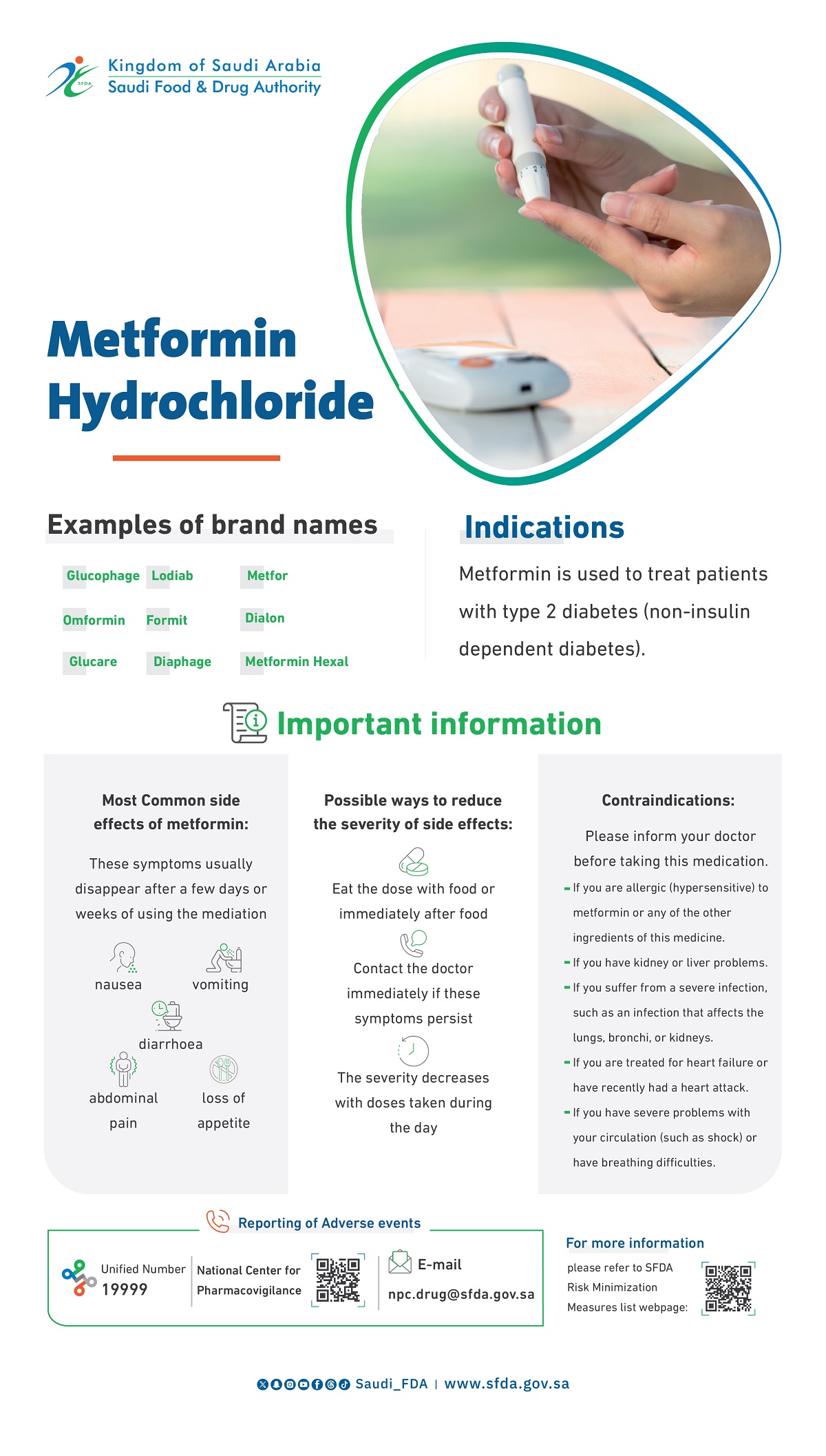
Metformin versus Insulin for the Treatment of Gestational Diabetes
Metformin versus Insulin for the Treatment of Gestational Diabetes
Metformin versus Insulin for the Treatment of Gestational Diabetes
2008-05-14
Metformin is a logical treatment for women with gestational diabetes mellitus, but randomized trials to assess the efficacy and safety of its use for this condition are lacking.
We randomly assigned 751 women with gestational diabetes mellitus at 20 to 33 weeks of gestation to open treatment with metformin (with supplemental insulin if required) or insulin. The primary outcome was a composite of neonatal hypoglycemia, respiratory distress, need for phototherapy, birth trauma, 5-minute Apgar score less than 7, or prematurity. The trial was designed to rule out a 33% increase (from 30% to 40%) in this composite outcome in infants of women treated with metformin as compared with those treated with insulin. Secondary outcomes included neonatal anthropometric measurements, maternal glycemic control, maternal hypertensive complications, postpartum glucose tolerance, and acceptability of treatment.
Results: Of the 363 women assigned to metformin, 92.6% continued to receive metformin until delivery and 46.3% received supplemental insulin. The rate of the primary composite outcome was 32.0% in the group assigned to metformin and 32.2% in the insulin group (relative risk, 1.00; 95% confidence interval, 0.90 to 1.10). More women in the metformin group than in the insulin group stated that they would choose to receive their assigned treatment again (76.6% vs. 27.2%, P<0.001). The rates of other secondary outcomes did not differ significantly between the groups. There were no serious adverse events associated with the use of metformin.
In women with gestational diabetes mellitus, metformin (alone or with supplemental insulin) is not associated with increased perinatal complications as compared with insulin. The women preferred metformin to insulin treatment.
Nejm , Volume 358:2003-2015,May 8,2008 ,Number 19