Post-Market Evaluation for the Safety and Effectiveness of Paclitaxel Coated Balloon and Paclitaxel Eluting Stent Devices
Post-Market Evaluation for the Safety and Effectiveness of Paclitaxel Coated Balloon and Paclitaxel Eluting Stent Devices
The safety of Paclitaxel eluting stent and Paclitaxel coated balloon has been evaluated extensively by international regulatory offices and specialized societies, due to the long term risks of the mortality following the application of them. FDA released a safety communication on the 17th of January 2019 about the potential for increased long-term mortality after use of paclitaxel-coated balloons and paclitaxel-eluting stents (collectively “paclitaxel-coated products”) to treat peripheral arterial disease (PAD) in the femoropopliteal artery.
Nevertheless, and considering the fact that there is an expanded market for Paclitaxel eluting stent and Paclitaxel coated balloon in Saudi Arabia, SFDA takes the initiative to evaluate the safety of Paclitaxel eluting stent and Paclitaxel coated balloon in light of the recent accumulation of clinical data.
1 Paclitaxel coated balloons and paclitaxel eluting stents needs
Peripheral artery disease (also called peripheral arterial disease) is a common circulatory problem in which narrowed arteries reduce blood flow to the limbs. Developing Peripheral artery disease means that there is no enough blood flow to the extremities usually the legs which result in leg pain when walking (claudication). Peripheral artery disease (PAD) is also likely to be a sign of a more widespread accumulation of fatty deposits in your arteries (atherosclerosis). This condition may be reducing blood flow to your heart and brain, as well as your legs [1]. One of the main symptoms of peripheral artery disease is the have leg pain when walking (claudication). Claudication symptoms include muscle pain or cramping in the legs or arms that's triggered by activity, such as walking, but disappears after a few minutes of rest. The location of the pain depends on the location of the clogged or narrowed artery. If peripheral artery disease progresses, pain may even occur when at rest or when the patients lying down (ischemic rest pain). Hence, the symptoms of PAD ranging from leg pain when walking (claudication) to tissue loss may result from Lower extremity PAD in the femoropopliteal segment which may in the end lead to amputation (critical limb ischemia)
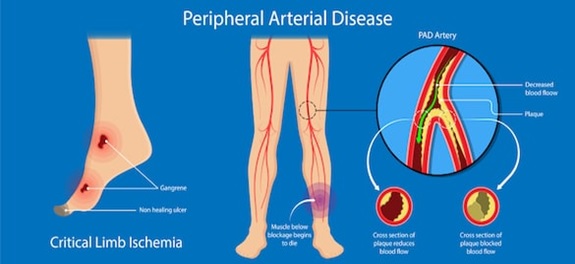
Figure1: Illustration of the peripheral arterial disease [2]
One of the prevalent condition that affect the aging population is the peripheral arterial disease (PAD). The prevalence of PAD is range from 3% to 10%. in people aged < 70 years and from 15% to 20% in people aged >70 years as estimated by several epidemiologic studies [3]. PAD contributes significantly to the morbidity and mortality of adults. In addition to that, PAD consider a significant economic burden The high rates of initial and repeat revascularization procedures is the main reason of the high hospitalization costs for patients with PAD . [83]
There are many endovascular therapies for PAD include percutaneous transluminal angioplasty (PTA), stenting, bypass surgery and atherectomy. These therapies are often associated with a high incidence of restenosis (i.e., re-narrowing of the treated vessel segment) due to a neointimal proliferation - migration of vascular smooth muscle cells primarily in the innermost layer of an artery or vein- in response to vessel injury. Over the past decade, drug-coated devices, including drug-coated balloons (DCB) and drug-eluting stents (DES), have been approved to treat de novo and restenotic lesions in the superficial femoral arteries (SFA) and proximal popliteal arteries (PPA) in PAD patients. One of these drug-coated devices is Paclitaxel eluting stent and Paclitaxel coated balloons
2 Paclitaxel coated balloons and paclitaxel eluting stents specification
2.1 Paclitaxel
The paclitaxel was developed at the beginning as an anti-cancer anti-proliferative agent. It is essentially insoluble in water, so for intravenous administration to cancer patients, it is dissolved in an oil base to form the drug Taxol. Then it has been introduced to be use for coronary stenting. Because paclitaxel is not soluble in water, it is not suitable alone for systemic therapy, but is more applicable for contact-based, local delivery, as in drug-eluting stents. One obvious example is its use as a local anti-proliferative to reduce restenosis. The main difference between paclitaxel as used in cancer treatment versus stenting is the dose. Paclitaxel-eluting stents administer tiny doses in comparison to Taxol in cancer treatment. Microtubules are responsible about cell division so paclitaxel works by stabilizing microtubules by rendering them nonfunctional. The drug has unique multifunctional effects that inhibit the restenotic process. It promotes the polymerization of stable nonfunctioning mictrotubules and thus inhibits all microtubule-dependent activities. As a result, it can inhibit multiple cellular components of the restenotic process. In addition, paclitaxel is highly lipophilic, which contributes to more efficient drug transfer off the stent and into the tissue. Lastly, paclitaxel has a dose-dependent inhibitory effect on smooth muscle cell proliferation. Because smooth muscle cells are more sensitive to paclitaxel than endothelial cells, it reduces restenosis without compromising the healing of the arterial wall [5].
The design of DCB and DES allow for an initial drug dose followed by a sustained cytostatic drug level to inhibit smooth muscle cell proliferation and neointimal growth, leading to reduced restenosis rates. The kinetics of drug release rely on several factors, including drug dose density, degree of coating crystallinity, the type of the carrier excipient, and total drug load. While increased drug crystallinity generally helps achieve higher tissue uptake and prolonged retention, an increased amorphous content usually facilitates a durable coating of the drug on the balloon during tracking of the device to the target lesion, Currently-marketed devices initially source similar crystalline paclitaxel, but the processing of individual devices likely results in differing ratios of amorphous and crystalline content. [6]
Paclitaxel eluting stent and Paclitaxel coated balloons may be combined with an excipient. The excipient enables uniform distribution of the drug on the device and facilitates drug transfer upon balloon inflation or stent implantation during contact with the endoluminal surface. The choice of an excipient impacts various device properties, including systemic drug loss during transit to the lesion site, tissue retention, drug release during inflation/implantation and local drug transfer. Microparticle formation from these coatings is often observed, which may embolize to the downstream systemic circulation. The amount of particulate formation varies depending on device design and processing [7].
2.2 Drug coated balloon vs Drug eluting stent
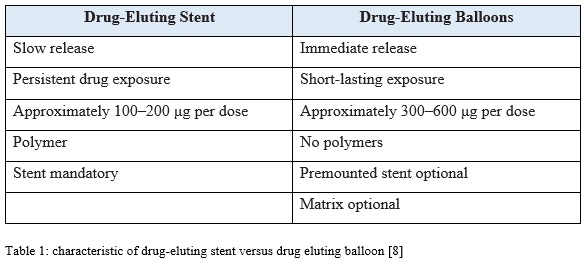
Drug-eluting balloons is deliver the drug on site with a precise control of the drug dosage. Therefore, there is not systemic exposure to the drug because the concentration is effective and sufficient. It has an advantage when compare with the stent which is possibility of a homogeneous drug transfer where as in the stent, the drug is only delivered at the contact site of the stent struts with the vessel wall. The stent struts cover approximately 15% stented vessel wall area, which result in low tissue concentrations of the ant proliferative agent in these areas
Paclitaxel eluting stent and Paclitaxel coated balloons are reported to be associated with the increase of long term mortality rate complications that impact patient’s safety. Literatures were reviewed to evaluate the pre-clinical safety for Paclitaxel eluting stent and Paclitaxel coated balloons which has been approved by FDA. It was shown that there was no evidence of potential device-related safety concerns. In addition, the literatures were reviewed for RCTs and meta‐analysis to evaluate the safety of Paclitaxel eluting stent and Paclitaxel coated balloons. It demonstrates that there is good safety profile for these devices, however, some of the longer‐term follow‐up RCTs have shown hints of increased late patient mortality with the use of Paclitaxel eluting stent and Paclitaxel coated balloons in the absence of obvious causal links [4]
Recently, there is a debate about the safety of Paclitaxel eluting stent and Paclitaxel coated balloon in term of long term mortality among international regulatory organizations. MHRA have formed an independent Expert Advisory Group (EAG) to review the available information on paclitaxel eluting stent and paclitaxel coated balloon after the concern that has been raised by Katsanos et al. The EAG group is made up of leading UK clinicians from specialist societies, including interventional radiology, vascular surgery and scientists with toxicology, medicines and statistical expertise. Their publication’s findings show that there is a possible increase in the mortality rate from 2 to 5 years in PAD patients treated with paclitaxel coated balloons and paclitaxel eluting stents compare with PAD patients treated with non-coated balloons or bare metal stents. The causal relationship for this observation has not been identified yet. This may reflect limitations in the way the data were analyzed. The devices have valid CE certificates and still remain on the UK market.
USFDA have issued 2 field safety notices in the database regarding Paclitaxel eluting stent and Paclitaxel coated balloon and all of them have been given recall class III. There are a number of paclitaxel-coated balloons or paclitaxel-eluting stents approved or under study for peripheral vascular use in the FDA is currently evaluating available long-term follow-up data to determine if there are any long-term risks associated with paclitaxel-coated products. This will include an evaluation of long-term follow-up data from studies that supported approval of paclitaxel-coated balloons or paclitaxel-eluting stents in the U.S. and other available data sets. This review will focus on causes of death, the paclitaxel dose delivered, and patient characteristics that may impact clinical outcomes. Additional statistical analyses will be performed to clarify the presence and magnitude of any long-term risks.
The safety and effectiveness of Paclitaxel Coated Balloon and Paclitaxel Eluting Stent Devices for the treatment of (PAD) were evaluated considering two main sections: the clinical paper review and the clinical experience review. While the first aims to review the published papers in the topic, the second will explore the opinions of other regulatory offices, international specialized societies, and most importantly the opinions of the local experts, represented. These two elements will be then used to draw an overall evaluation regarding the risk of increasing the long-term mortality rate of paclitaxel eluting stent and paclitaxel coated balloons, which will be demonstrated form of current-evidence-based recommendations about the device safety.
Part 1: Clinical paper review
- An overview of the search criteria
Table 2 specify the search criteria, it specify the inclusion criteria and the quality measures for the review. As a result, 91 articles were acquired, and screened first for duplication, and then through scanning the abstract as guided by the clarified inclusion criteria.
Lastly, 9 specific articles were obtained and read in full. Figure 2 shows a schematic representation of the search findings on evaluating the safety of Paclitaxel eluting stent and Paclitaxel coated balloons.
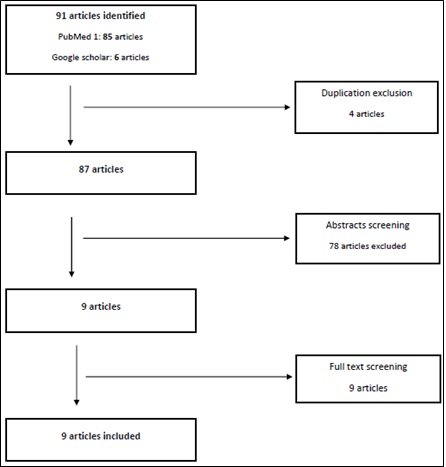
Figure 2: Schematic representation of the search findings.
- II) Summary of the paper review findings
- A number of 9 studies were included after applying the inclusion criteria and the quality measures, which include 6 RCTs and 3 meta-analyses that compare the mortality rate of the applying the standard PTA and paclitaxel coated devices.
- Figure 3 shows a comparison of the mortality rate of standard PTA and paclitaxel coated devices at a follow up period of 2 years and 5 years. The major observation suggests a significant correlation between morality rate and time. Paclitaxel coated devices is seen to have higher mortality rate than standard PTA which were seen to be 1.7% at a follow up of 2 years and at a follow up of 5 years the difference was seen to be increased significantly at a difference in the mortality rate of 5.9%.
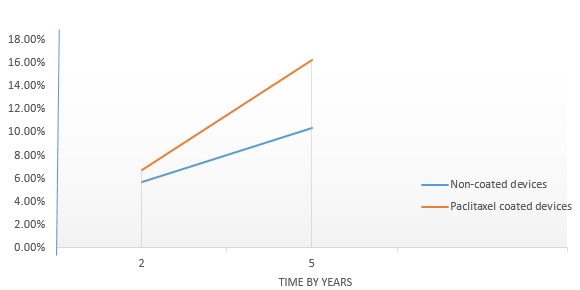
Figure 3: A comparison of the mortality rate of non-coated devices and paclitaxel coated devices.
Part 2: The opinion of global specialized societies and associations in Paclitaxel coated balloons and paclitaxel eluting stents
American heart Association has conducted a systematic review and meta‐analysis of RCTs to investigate the safety issue of paclitaxel coated balloons and paclitaxel eluting stents in the femoral and/or popliteal arteries. The primary safety measure was all‐cause patient death. A random effects model was used for the risk ratios and risk differences. 28 RCTs with 4663 patients were analyzed. All‐cause patient death was similar between paclitaxel‐coated devices and control arms at 1 year (28 RCTs with 4432 cases). All‐cause death at 2 years (12 RCTs with 2316 cases) was significantly increased in the case of paclitaxel versus control. All‐cause death up to 5 years (3 RCTs with 863 cases) increased further in the case of paclitaxel Meta‐regression showed a significant relationship between the absolute risk of death and the exposure to paclitaxel (dose‐time product) (0.4±0.1% excess risk of death per paclitaxel mg‐year; P<0.001). Trial sequential analysis excluded false‐positive findings with 99% certainty (2‐sided α, 1.0%).
Part 3: Saudi user experience
Saudi experts have been consulted about the risk of increasing the mortality rate for patients who treated with paclitaxel eluting stent and paclitaxel coated balloon, in an evaluation check that contains the following questions:
- Q1: Based on your experience, what is your opinion about the safety of paclitaxel eluting stent and paclitaxel coated balloon when used for PAD in term of long-term mortality (2-5 years)? They have been given four choices to answer this question as safe, safe, but more studies are needed, there is safety issue, but I will continue using it, and there is safety issue, I stopped (or I will stop) using it.
- Q2: Do you have any comments you want to add about the raised issue of safety of paclitaxel Coated Balloon and paclitaxel Eluting Stent Devices when used for PAD. in term of long term mortality (2-5 years)?
The results: Thirty-one experts have been consulted and their answer for the first question is illustrated in figure 4, where the majority (74%) think that the device is safe, but more studies are needed to evaluated the device safety at the long-term.
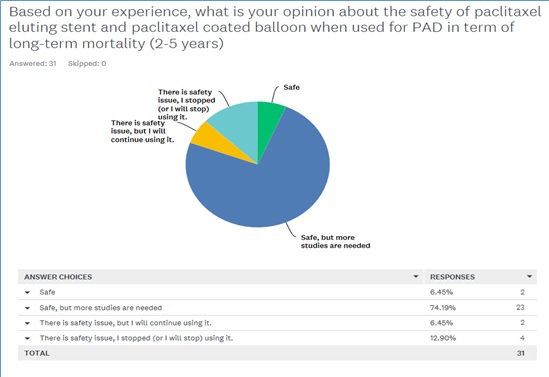
Figure 4: Saudi expertise opinion about the safety of paclitaxel eluting stent and paclitaxel-coated balloon.
However, and with respect to the second question, the majority of the responders believe that the paclitaxel eluting stent and paclitaxel-coated balloon when used for PAD are safe and effective and the benefit of using them outweigh the risk. Nevertheless, some of the experts have different opinion regarding the reliability of the original study that highlight the case-safety signal.
Overall Conclusion
The objective of this post-market evaluation is to evaluate the safety of paclitaxel coated balloon and paclitaxel eluting stent in term of long term mortality comparing with non-coated devices. The literature review shows that there was a good evidence that using paclitaxel‐coated devices in the femoropopliteal artery was related to significantly long term increased risk of death comparing with non-coated devices. International regulatory organizations mentioned that there is a possible increase in the mortality rate from 2 to 5 years in PAD patients treated with paclitaxel coated balloons and paclitaxel eluting stents compare with PAD patients treated with non-coated devices. The global societies and associations have conducted systematic review and meta‐analysis of RCTs to investigate the safety issue of paclitaxel coated balloons and paclitaxel eluting stents in the femoral and/or popliteal arteries. They come up with that, there was a relationship between the risk of long-term mortality and the exposure to paclitaxel. Saudi user experience has been taken about the safety issue of paclitaxel-coated devices and they believe that, the devices are safe however, more studies are needed. To conclude that, more studies are needed to evaluate the safety of paclitaxel eluting stent and paclitaxel coated balloon in term of long-term mortality comparing with non-coated devices.
Considering the results of the post-market evaluation of the safety and effectiveness of Paclitaxel Coated Balloon and Paclitaxel Eluting Stent Devices for the treatment of (PAD), the following actions were taken by SFDA:
- Request a post-market follow up studies from the manufacturers confirming the safety of the devices.
- Publish a safety communication letter including the following information:
-
“SFDA would like to draw your attention to the meta-analysis studies that show an increase in late mortality rate (2-5 years) after the application of paclitaxel coated balloon and paclitaxel eluting stent to treat patients with peripheral arterial disease (PAD). However, there are limitations in these meta-analyses, the benefits of paclitaxel-coated devices (e.g., reduced reinterventions) should be considered in individual patients along with potential risks (e.g., late mortality)”.
Recommendations for Healthcare Providers
- Continue monitoring patients who have been treated with paclitaxel –coated balloon and paclitaxel eluting stents per the current standards of the care for longer than 2 years.
- Report any incident / adverse event or suspected adverse events experienced with the use of paclitaxel-coated balloons and paclitaxel-eluting stents through National center for medical devices reporting (NCMDR) https://ncmdr.sfda.gov.sa, or the Saudi Vigilance System https://ade.sfda.gov.sa/, to help the SFDA identify and better understand the risk associated with the devices.
Grateful thanks to Eng. Abdulrahman Alanazi for designing, reviewing the up to date articles, and writing up the context of this study. Eng. Bader Aloufi verified the study methodology and supervised the study progress. With the appreciation to Rashed Abu Haimed, for drafting this summary and the post-market clinical evaluation team for their efforts in conducting this work.
For further information or inquiries related to this study, you may contact us at: cia.md@sfda.gov.sa
|
[1] |
M. C. Staff, "Peripheral artery disease (PAD)," Mayo Foundation for Medical Education and Research (MFMER). , 2019. [Online]. Available: https://www.mayoclinic.org/diseases-conditions/peripheral-artery-disease/symptoms-causes/syc-20350557. [Accessed 19 8 2019]. |
|
[2] |
"Peripheral artery disease images," shutterstock , [Online]. Available: https://www.shutterstock.com/search/Peripheral+artery+disease. [Accessed 22 8 2019]. |
|
[3] |
Candy N1, Ng E1, Velu R2., "Paclitaxel-coated balloon reduces target lesion revascularization compared with standard balloon angioplasty," J Vasc Surg. 2017 , vol. 65, no. 2, pp. 558-570., 2017. |
|
[4] |
Z. AlSabelah, K. Alghamdi and M. AlHayan, "Technology: Paclitaxel Coated Balloon, Paclitaxel Eluting Stent For The Treatment of Peripheral Arterial Disease (PAD)," SFDA, Riyadh, 2019. |
|
[5] |
M. Alan W. Heldman, "Drug-eluting Stent Solutions: Examining the Anti-Proliferative Drug Paclitaxel," cardiovascular learning network, [Online]. Available: https://www.cathlabdigest.com/articles/Drug-eluting-Stent-Solutions-Examining-Anti-Proliferative-Drug-Paclitaxel. [Accessed 9 10 2019]. |
|
[6] |
Granada JF1, Stenoien M2, Buszman PP1, Tellez A1, Langanki D2, Kaluza GL1, Leon MB3, Gray W3, Jaff MR4, Schwartz RS5., "Mechanisms of tissue uptake and retention of paclitaxel-coated balloons: impact on neointimal proliferation and healing," Open Heart. , vol. 1, no. 1, 2014. |
|
[7] |
Kenneth Ouriel, Mark A. Adelman, Kenneth Rosenfield, Dierk Scheinert, Marianne Brodmann, Constantino Peña, Patrick Geraghty, Arthur Lee, Roseann White and Daniel G. Clair, "Safety of Paclitaxel-Coated Balloon Angioplasty for Femoropopliteal Peripheral Artery Disease," JACC: Cardiovascular Interventions, 2019. |
|
[8] |
By Jos C. Van Den Berg, MD, PhD, "Drug-Eluting Balloons in Below-the-Knee Applications," 2010. |
|
[9] |
Zhen Y1, Chang Z1, Wang C1, Liu Z1, Zheng J2., "Directional Atherectomy with Antirestenotic Therapy for Femoropopliteal Artery Disease: A Systematic Review and Meta-Analysis.," J Vasc Interv Radiol., vol. 30, no. 10, pp. 1586-1592, 2019. |
|
[10] |
Mustapha JA1, Brodmann M, Geraghty PJ, Saab F, Settlage RA, Jaff MR; Lutonix BTK Study Investigators., "Drug-Coated vs Uncoated Percutaneous Transluminal Angioplasty in Infrapopliteal Arteries: Six-Month Results of the Lutonix BTK Trial.," J Invasive Cardiol. 2019 Aug;31(8):205-211., vol. 31, no. 8, pp. 205-211, 2019. |
|
[11] |
Soga Y1, Fujihara M2, Tomoi Y1, Iida O3, Ishihara T3, Kawasaki D4, Ando K1., "One-Year Late Lumen Loss between A Polymer-Coated Paclitaxel-Eluting Stent (Eluvia) and a Polymer-Free Paclitaxel-Coated Stent (Zilver PTX) for Femoropopliteal Disease.," Atheroscler Thromb. , vol. 29, 2019. |
|
[12] |
Tsukiyama Y1, Shinke T2,3, Ishihara T4, Otake H1, Terashita D1, Kozuki A5, Fukunaga M6, Zen K7, Horimatsu T6, Fujii K6, Shite J5, Uematsu M4, Takahara M8, Iida O4, Nanto S9, Hirata KI1., "Vascular response to paclitaxel-eluting nitinol self-expanding stent in superficial femoral artery lesions: post-implantation angioscopic findings from the SHIMEJI trial (Suppression of vascular wall Healing after IMplantation of drug Eluting peripheral s," Int J Cardiovasc Imaging. , vol. 35, no. 10, pp. 1777-1784, 2019. |
|
[13] |
Du X1, Wang F2, Wu DM3, Zhang MH1, Jia X1, Zhang JW4, Zhuang BX5, Zhao Y6, Guo PF7, Bi W8, Fu WG9, Guo W1, Wang SM10., "Comparison between paclitaxel-coated balloon and standard uncoated balloon in the treatment of femoropopliteal long lesions in diabetics.," Medicine (Baltimore)., vol. 98, no. 13, 2019. |
|
[14] |
Albrecht T1,2, Schnorr B3, Kutschera M3, Waliszewski MW4,5., "Two-Year Mortality After Angioplasty of the Femoro-Popliteal Artery with Uncoated Balloons and Paclitaxel-Coated Balloons-A Pooled Analysis of Four Randomized Controlled Multicenter Trials," Cardiovasc Intervent Radiol., vol. 42, no. 7, pp. 949-955, 2019. |
|
[15] |
Stabile E1, Gerardi D1, Magliulo F1, Zhelev D2, Chervenkoff V3, Taeymans K4, Kotasov D2, Goverde P4, Giugliano G5, Trimarco B1, Esposito G1., "One-Year Clinical Outcomes of the Legflow Drug-Coated Balloon for the Treatment of Femoropopliteal Occlusions Registry.," J Endovasc Ther., vol. 26, no. 1, pp. 26-30, 2019. |
|
[16] |
Dake MD1, Ansel GM2, Jaff MR2, Ohki T2, Saxon RR2, Smouse HB2, Machan LS2, Snyder SA2, O'Leary EE2, Ragheb AO2, Zeller T2; Zilver PTX Investigators., "Durable Clinical Effectiveness With Paclitaxel-Eluting Stents in the Femoropopliteal Artery: 5-Year Results of the Zilver PTX Randomized Trial.," Circulation. , vol. 133, no. 15, pp. 1472-83, 2016`. |
|
[17] |
Bracale UM1, Di Filippo M1, De Capua A1, Vanni L1, Narese D2, Pecoraro F3, Giribono AM1,4, Bracale R4., "Treatment of de novo femoro-popliteal lesions with a new Drug Coated Balloon: early experience of a single Center in the first 50 patients.," Transl Med UniSa. 2019 Jan 20;18:3-8. eCollection 2018 Nov., vol. 20, pp. 3-8, 2019. |
|
[18] |
Kichikawa K1, Ichihashi S2, Yokoi H3, Ohki T4, Nakamura M5, Komori K6, Nanto S7, O'Leary EE8, Lottes AE8, Snyder SA8, Dake MD9., "Zilver PTX Post-market Surveillance Study of Paclitaxel-Eluting Stents for Treating Femoropopliteal Artery Disease in Japan: 2-Year Results.," Cardiovasc Intervent Radiol.., vol. 42, no. 3, pp. 358-364, 2019. |
|
[19] |
Gray WA1, Keirse K2, Soga Y3, Benko A4, Babaev A5, Yokoi Y6, Schroeder H7, Prem JT8, Holden A9, Popma J10, Jaff MR11, Diaz-Cartelle J12, Müller-Hülsbeck S13; IMPERIAL investigators., "A polymer-coated, paclitaxel-eluting stent (Eluvia) versus a polymer-free, paclitaxel-coated stent (Zilver PTX) for endovascular femoropopliteal intervention (IMPERIAL): a randomised, non-inferiority trial.," Lancet.., vol. 27, pp. 1541-1551, 2018. |
|
[20] |
Dugas TR1, Brewer G2, Longwell M2, Fradella T2, Braun J2, Astete CE2, Jennings MH1, Sabliov CM2., "Nanoentrapped polyphenol coating for sustained drug release from a balloon catheter.," J Biomed Mater Res B Appl Biomater. , vol. 107, no. 3, pp. 646-651., 2019. |
|
[21] |
Brodmann M1, Zeller T2, Christensen J3, Binkert C4, Spak L5, Schröder H6, Righini P7, Nano G7, Tepe G8., "Real-world experience with a Paclitaxel-Coated Balloon for the treatment of atherosclerotic infrainguinal arteries: 12-month interim results of the BIOLUX P-III registry first year of enrolment," J Vasc Bras. 2017 Oct-Dec;16(4):276-284. doi: 10.1590/1677-5449.007317., vol. 16, no. 4, pp. :276-284., 2017. |
|
[22] |
Bisdas T1, Beropoulis E2, Argyriou A2, Torsello G2, Stavroulakis K2., "1-Year All-Comers Analysis of the Eluvia Drug-Eluting Stent for Long Femoropopliteal Lesions After Suboptimal Angioplasty.," JACC Cardiovasc Interv. , vol. 11, no. 10, pp. 957-966, 2018. |
|
[23] |
Schneider PA1, Laird JR2, Tepe G2, Brodmann M2, Zeller T2, Scheinert D2, Metzger C2, Micari A2, Sachar R2, Jaff MR2, Wang H2, Hasenbank MS2, Krishnan P2; IN.PACT SFA Trial Investigators., "Treatment Effect of Drug-Coated Balloons Is Durable to 3 Years in the Femoropopliteal Arteries: Long-Term Results of the IN.PACT SFA Randomized Trial.," Circ Cardiovasc Interv. , vol. 11, no. 1, 2018. |
|
[24] |
Steiner S1, Willfort-Ehringer A2, Sievert H3, Geist V4, Lichtenberg M5, Del Giudice C6, Sauguet A7, Diaz-Cartelle J8, Marx C9, Ströbel A9, Schult I10, Scheinert D11; RANGER SFA Investigators., "12-Month Results From the First-in-Human Randomized Study of the Ranger Paclitaxel-Coated Balloon for Femoropopliteal Treatment.," JACC Cardiovasc Interv. 2018 May 28;11(10):934-941. doi: 10.1016/j.jcin.2018.01.276. Epub 2018 May 2., vol. 11, no. 10, pp. :934-941., 2018. |
|
[25] |
Ding Y1, Zhou M1, Wang Y2, Cai L1, Shi Z3., "Comparison of Drug-Eluting Stent with Bare-Metal Stent Implantation in Femoropopliteal Artery Disease: A Systematic Review and Meta-Analysis.," Ann Vasc Surg. , vol. 50, pp. 96-105. , 2018. |
|
[26] |
Elens M1, Verhelst R1, Possoz J1, Mastrobuoni S1, Lacroix V1, Astarci P , "Short-Term Results of Eluvia™ Paclitaxel-Eluting Stent in External Iliac and Femoropopliteal Lesions.," Surgical Technology International, 01 Nov 2017, 31:, vol. 31, pp. 162-167, 2017. |
|
[27] |
Lichtenberg M1, von Bilderling P2, Ranft J3, Niemöller K3, Grell H4, Briner L5, Saucy F5, Rassaf T6, Breuckmann F7., "Treatment of femoropopliteal atherosclerotic lesions using the ranger paclitaxel-coated balloon catheter: 12-month results from an all-comers registry.," J Cardiovasc Surg (Torino). , vol. 59, no. 1, pp. 45-50, 2018. |
|
[28] |
Müller-Hülsbeck S1, Keirse K2, Zeller T3, Schroë H4, Diaz-Cartelle J5., "Long-Term Results from the MAJESTIC Trial of the Eluvia Paclitaxel-Eluting Stent for Femoropopliteal Treatment: 3-Year Follow-up.," Cardiovasc Intervent Radiol, vol. 40, no. 12, pp. 1832-1838., 2017. |
|
[29] |
Tepe G1, Zeller T, Albrecht T, Heller S, Schwarzwälder U, Beregi JP, Claussen CD, Oldenburg A, Scheller B, Speck U., "Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg.," N Engl J Med. , vol. 358(, no. 7, pp. 689-99., 2008. |
|
[30] |
Zeller T1, Langhoff R2, Rocha-Singh KJ2, Jaff MR2, Blessing E2, Amann-Vesti B2, Krzanowski M2, Peeters P2, Scheinert D2, Torsello G2, Sixt S2, Tepe G2; , "Directional Atherectomy Followed by a Paclitaxel-Coated Balloon to Inhibit Restenosis and Maintain Vessel Patency: Twelve-Month Results of the DEFINITIVE AR Study.," Circ Cardiovasc Interv. 2017 Sep;10(9). , vol. 10, no. 9, 2017. |
|
[31] |
Deloge C1, Boesmans E1, Van Damme H1, Defraigne JO1., "Revascularization of the superficial femoral artery with paclitaxel-coated balloon for claudication.," Acta Chir Belg.., vol. 118, no. 1, pp. 42-47, 2018 . |
|
[32] |
Amendt K1, Beschorner U2, Waliszewski M3,4, Sigl M1, Langhoff R5, Thalwitzer J6, Redlich U7, Vogel B8, Härtel D9, Zeller T2., "First clinical experience with the Multi-LOC multiple stent delivery system for focal stenting in long femoro-popliteal lesions.," Vasa. , vol. 46, no. 6, pp. 452-461., 2017 . |
|
[33] |
Schroeder H1, Meyer DR2, Lux B3, Ruecker F4, Martorana M4, Miller LE5, Duda S4., "A Pilot Study of Femoropopliteal Artery Revascularisation with a Low Dose Paclitaxel Coated Balloon: Is Predilatation Necessary?," Eur J Vasc Endovasc Surg., vol. 54, no. 3, pp. 348-355. , 2017 . |
|
[34] |
Laird JA1, Schneider PA2, Jaff MR3, Brodmann M4, Zeller T5, Metzger DC6, Krishnan P7, Scheinert D8, Micari A9, Wang H10, Masters M10, Tepe G11., "Long-Term Clinical Effectiveness of a Drug-Coated Balloon for the Treatment of Femoropopliteal Lesions.," Circ Cardiovasc Interv., vol. 12, no. 6, 2019. |
|
[35] |
Tepe G1, Gögebakan Ö2, Redlich U3, Tautenhahn J3, Ricke J4, Halloul Z4, Meyer DR5, Waliszewski M6, Schnorr B7, Zeller T8, Müller-Hülsbeck S9, Ott I10, Albrecht T2., "Angiographic and Clinical Outcomes After Treatment of Femoro-Popliteal Lesions with a Novel Paclitaxel-Matrix-Coated Balloon Catheter.," Cardiovasc Intervent Radiol. , vol. 40, no. 10, pp. 1535-1544, 2017. |
|
[36] |
Bausback Y1, Willfort-Ehringer A2, Sievert H3,4, Geist V5, Lichtenberg M6, Del Giudice C7, Sauguet A8, Diaz-Cartelle J9, Marx C10, Ströbel A10, Schult I11, Scheinert D1; RANGER SFA Investigators., "Six-Month Results From the Initial Randomized Study of the Ranger Paclitaxel-Coated Balloon in the Femoropopliteal Segment.," J Endovasc Ther., vol. 24, no. 4, pp. 459-467., 2017. |
|
[37] |
Ogawa Y1, Yokoi H2, Ohki T3, Kichikawa K4, Nakamura M5, Komori K6, Nanto S7, O'Leary EE8, Lottes AE8, Saunders AT8, Dake MD9., "Impact of Chronic Renal Failure on Safety and Effectiveness of Paclitaxel-Eluting Stents for Femoropopliteal Artery Disease: Subgroup Analysis from Zilver PTX Post-Market Surveillance Study in Japan.," Cardiovasc Intervent Radiol. , vol. 40, no. 1, pp. 1669-1677., 2017. |
|
[38] |
Gasior P1, Cheng Y1, Valencia AF1, McGregor J1, Conditt GB1, Kaluza GL1, Granada JF2., "Impact of Fluoropolymer-Based Paclitaxel Delivery on Neointimal Proliferation and Vascular Healing: A Comparative Peripheral Drug-Eluting Stent Study in the Familial Hypercholesterolemic Swine Model of Femoral Restenosis.," Circ Cardiovasc Interv. , vol. 10, no. 5, 2017. |
|
[39] |
Vent PA1, Kaladji A2, Davaine JM1, Guyomarch B3, Chaillou P1, Costargent A1, Quillard T4, Gouëffic Y5., "Bare Metal Versus Paclitaxel-Eluting Stents for Long Femoropopliteal Lesions: Prospective Cohorts Comparison Using a Propensity Score-Matched Analysis.," Ann Vasc Surg. , vol. 43, pp. 166-175. , 2017. |
|
[40] |
Miki K1, Fujii K2, Shibuya M2, Fukunaga M3, Imanaka T2, Kawai K2, Tamaru H2, Sumiyoshi A2, Nishimura M2, Horimatsu T2, Saita T2, Yoshihara N2, Kimura T4, Honda Y4, Fitzgerald PJ4, Masuyama T2, Ishihara M5., "Impact of stent diameter on vascular response after self-expanding paclitaxel-eluting stent implantation in the superficial femoral artery.," J Cardiol. , vol. 40, no. 4, pp. 346-352., 2017. |
|
[41] |
Cioppa A1, Stabile E, Salemme L, Popusoi G, Pucciarelli A, Iacovelli F, Arcari A, Coscioni E, Trimarco B, Esposito G, Tesorio T., "Combined use of directional atherectomy and drug-coated balloon for the endovascular treatment of common femoral artery disease: immediate and one-year outcomes.," EuroIntervention. , vol. 12, no. 14, pp. 1789-1794., 2017. |
|
[42] |
Mehrotra S1, Paramasivam G2, Mishra S3., "Paclitaxel-Coated Balloon for Femoropopliteal Artery Disease.," Curr Cardiol Rep. , vol. 19, no. 2, 2017. |
|
[43] |
Micari A1, Brodmann M2, Keirse K3, Peeters P4, Tepe G5, Frost M6, Wang H7, Zeller T8; IN.PACT Global Study Investigators., "Drug-Coated Balloon Treatment of Femoropopliteal Lesions for Patients With Intermittent Claudication and Ischemic Rest Pain: 2-Year Results From the IN.PACT Global Study.," JACC Cardiovasc Interv. , vol. 11, no. 10, pp. :945-953., 2018. |
|
[44] |
Zen K1, Takahara M2, Iida O3, Soga Y4, Kawasaki D5, Nanto S6, Yokoi H7, Matoba S8; ZEPHYR Investigators., "Drug-eluting stenting for femoropopliteal lesions, followed by cilostazol treatment, reduces stent restenosis in patients with symptomatic peripheral artery disease.," J Vasc Surg. , vol. 65, no. 3, pp. 720-725., 2017. |
|
[45] |
Lake E1, Twigg M1, Farquharson F1., "Acute hypersensitivity reaction to femoral drug-coated balloons.," Vasa. , vol. 46, no. 3, pp. 223-225, 2017. |
|
[46] |
Debing E1, Aerden D2, Vanhulle A2,3, Gallala S2, von Kemp K2; TRIAL Investigators., "Paclitaxel-coated versus plain old balloon angioplasty for the treatment of infrainguinal arterial disease in diabetic patients: the Belgian diabetic IN.PACT Trial.," J Cardiovasc Surg (Torino)., vol. 58, no. 4, pp. 528-534., 2017. |
|
[47] |
Jia X1, Zhang J2, Zhuang B3, Fu W4, Wu D5, Wang F6, Zhao Y7, Guo P8, Bi W9, Wang S10, Guo W11., "Acotec Drug-Coated Balloon Catheter: Randomized, Multicenter, Controlled Clinical Study in Femoropopliteal Arteries: Evidence From the AcoArt I Trial.," JACC Cardiovasc Interv., vol. 9, no. 18, pp. 1941-9, 2016. |
|
[48] |
Iida O1, Takahara M2, Soga Y3, Hirano K4, Yamauchi Y5, Zen K6, Yokoi H7, Uematsu M8; ZEPHYR investigators., "Incidence and its characteristics of repetition of reintervention after drug-eluting stent implantation for femoropopliteal lesion.," J Vasc Surg. , vol. 64, no. 6, pp. 1691-1695, 2016. |
|
[49] |
Müller-Hülsbeck S1, Keirse K2, Zeller T3, Schroë H4, Diaz-Cartelle J5., "Twelve-Month Results From the MAJESTIC Trial of the Eluvia Paclitaxel-Eluting Stent for Treatment of Obstructive Femoropopliteal Disease.," J Endovasc Ther. , vol. 23, no. 5, pp. 701-7., 2016. |
|
[50] |
Micari A1, Vadalà G2, Castriota F3, Liso A4, Grattoni C3, Russo P5, Marchese A6, Pantaleo P7, Roscitano G2, Cesana BM8, Cremonesi A3., "1-Year Results of Paclitaxel-Coated Balloons for Long Femoropopliteal Artery Disease: Evidence From the SFA-Long Study.," JACC Cardiovasc Interv. , vol. 9, no. 9, pp. 950-6., 2016. |
|
[51] |
Albrecht T1, Waliszewski M2,3, Roca C4,5, Redlich U6, Tautenhahn J6, Pech M7, Halloul Z8, Gögebakan Ö9, Meyer DR10, Gemeinhardt I11, Zeller T12, Müller-Hülsbeck S13, Ott I14, Tepe G15., "Two-Year Clinical Outcomes of the CONSEQUENT Trial: Can Femoropopliteal Lesions be Treated with Sustainable Clinical Results that are Economically Sound?," Cardiovasc Intervent Radiol. , vol. 41, no. 7, pp. 1008-1014., 2018. |
|
[52] |
Yokoi H1, Ohki T2, Kichikawa K3, Nakamura M4, Komori K5, Nanto S6, O'Leary EE7, Lottes AE7, Snyder SA7, Dake MD8., "Zilver PTX Post-Market Surveillance Study of Paclitaxel-Eluting Stents for Treating Femoropopliteal Artery Disease in Japan: 12-Month Results.," JACC Cardiovasc Interv, vol. 9, no. 3, pp. :271-277. , 2016. |
|
[53] |
Katsanos K1, Spiliopoulos S2, Paraskevopoulos I3, Diamantopoulos A3, Karnabatidis D2., "Systematic Review and Meta-analysis of Randomized Controlled Trials of Paclitaxel-Coated Balloon Angioplasty in the Femoropopliteal Arteries: Role of Paclitaxel Dose and Bioavailability.," J Endovasc Ther., vol. 23, no. 2, pp. 356-70., 2016. |
|
[54] |
Werner M1, Schmidt A2, Scheinert S2, Banning-Eichenseer U2, Ulrich M2, Bausback Y2, Steiner S2, Scheinert D2., "Evaluation of the Biodegradable Igaki-Tamai Scaffold After Drug-Eluting Balloon Treatment of De Novo Superficial Femoral Artery Lesions: The GAIA-DEB Study.," J Endovasc Ther., vol. 23, no. 1, pp. ):92-7. , 2016. |
|
[55] |
Zeller T1, Beschorner U2, Pilger E3, Bosiers M4, Deloose K4, Peeters P5, Scheinert D6, Schulte KL7, Rastan A8, Brodmann M3., "Paclitaxel-Coated Balloon in Infrapopliteal Arteries: 12-Month Results From the BIOLUX P-II Randomized Trial (BIOTRONIK'S-First in Man study of the Passeo-18 LUX drug releasing PTA Balloon Catheter vs. the uncoated Passeo-18 PTA balloon catheter in subjec," JACC Cardiovasc Interv. 2015 , vol. 8, no. 12, pp. 1614-22. , 2015. |
|
[56] |
Davaine JM1, Querat J2, Kaladji A2, Guyomarch B3, Chaillou P2, Costargent A2, Quillard T4, Gouëffic Y5., "Treatment of TASC C and D Femoropoliteal Lesions with Paclitaxel eluting Stents: 12 month Results of the STELLA-PTX Registry.," Eur J Vasc Endovasc Surg. , vol. 50, no. 5, pp. 631-7. , 2015. |
|
[57] |
Miki K, Fujii K, Fukunaga M, Nishimura M, Horimatsu T, Saita T, Sumiyoshi A, Tamaru H, Imanaka T, Shibuya M, Naito Y, Masuyama T, Ishihara M., "Strut Coverage After Paclitaxel-Eluting Stent Implantation in the Superficial Femoral Artery.," JACC Cardiovasc Imaging., vol. 9, no. 6, pp. 753-5., 2016. |
|
[58] |
Tepe G1, Schnorr B2, Albrecht T3, Brechtel K4, Claussen CD4, Scheller B5, Speck U2, Zeller T6., "Angioplasty of femoral-popliteal arteries with drug-coated balloons: 5-year follow-up of the THUNDER trial.," JACC Cardiovasc Interv. , vol. 8, no. 1, pp. 102-8, 2015. |
|
[59] |
Gongora CA1, Shibuya M1, Wessler JD2, McGregor J1, Tellez A3, Cheng Y1, Conditt GB1, Kaluza GL1, Granada JF4., "Impact of Paclitaxel Dose on Tissue Pharmacokinetics and Vascular Healing: A Comparative Drug-Coated Balloon Study in the Familial Hypercholesterolemic Swine Model of Superficial Femoral In-Stent Restenosis.," JACC Cardiovasc Interv. , vol. 8, no. 8, pp. :1115-1123., 2015. |
|
[60] |
Iida O1, Takahara M2, Soga Y3, Nakano M4, Yamauchi Y5, Zen K6, Kawasaki D7, Nanto S8, Yokoi H9, Uematsu M10; ZEPHYR Investigators., "1-Year Results of the ZEPHYR Registry (Zilver PTX for the Femoral Artery and Proximal Popliteal Artery): Predictors of Restenosis.," JACC Cardiovasc Interv. 2015 Jul;8(8):, vol. 8`, no. 8, pp. 1105-1112., 2015. |
|
[61] |
Konstantinos KatsanosStavros Spiliopoulos Panagiotis Kitrou-Miltiadis KrokidisDimitrios Karnabatidis, "Risk of Death Following Application of Paclitaxel‐Coated Balloons and Stents in the Femoropopliteal Artery of the Leg: A Systematic Review and Meta‐Analysis of Randomized Controlled Trials," Journal of the American Heart Association. , 2018. |
|
[62] |
Mayor S1., "Angioplasty with paclitaxel coated balloon reduces restenosis in peripheral artery disease.," BMJ. , 2015. |
|
[63] |
Dake MD1, et al., "Sustained safety and effectiveness of paclitaxel-eluting stents for femoropopliteal lesions: 2-year follow-up from the Zilver PTX randomized and single-arm clinical studies.," J Am Coll Cardiol. , vol. 61, no. 24, pp. 2417-2427., 2013. |
|
[64] |
Scheinert D1, et al., "Paclitaxel-releasing balloon in femoropopliteal lesions using a BTHC excipient: twelve-month results from the BIOLUX P-I randomized trial.," J Endovasc Ther., vol. 22, no. 1, pp. 14-21., 2015. |
|
[65] |
Schroeder H1, et al., "Two-year results of a low-dose drug-coated balloon for revascularization of the femoropopliteal artery: outcomes from the ILLUMENATE first-in-human study.," Catheter Cardiovasc Interv, vol. 86, no. 2, pp. 278-86., 2015. |
|
[66] |
Tepe G1, et al., "Angioplasty of femoral-popliteal arteries with drug-coated balloons: 5-year follow-up of the THUNDER trial.," JACC Cardiovasc Interv. , vol. 8, no. 1, pp. 102-8. , 2015. |
|
[67] |
Tepe G1, Laird J2, Schneider P1, Brodmann M1, Krishnan P1, Micari A1, Metzger C1, Scheinert D1, Zeller T1, Cohen DJ1, Snead DB1, Alexander B1, Landini M1, Jaff MR1; IN.PACT SFA Trial Investigators., "Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial.," Circulation., vol. 131, no. 5, pp. 495-502., 2015. |
|
[68] |
Soga Y1, Inoue K2, Kuma S3., "Pathological findings of late stent thrombosis after paclitaxel-eluting stent implantation for superficial femoral artery disease.," J Cardiol Cases., vol. 11, no. 2, pp. :39-41. , 2014. |
|
[69] |
Gouëffic Y1, Kaladji A, Guyomarch B, Montagne C, Fairier D, Gestin S, Riche VP, Vent PA, Chaillou P, Costargent A, Patra P., "Bare metal stent versus paclitaxel eluting stent for intermediate length femoropopliteal arterial lesions (BATTLE trial): study protocol for a randomized controlled trial.," Trials. , 2014. |
|
[70] |
Tellez A1, Dattilo R, Mustapha JA, Gongora CA, Hyon CM, Palmieri T, Rousselle S, Kaluza GL, Granada JF., "Biological effect of orbital atherectomy and adjunctive paclitaxel-coated balloon therapy on vascular healing and drug retention: early experimental insights into the familial hypercholesterolaemic swine model of femoral artery stenosis.," EuroIntervention. , vol. 10, no. 8, pp. 1002-8., 2014. |
|
[71] |
Ohki T1, et al, "Two-year analysis of the Japanese cohort from the Zilver PTX randomized controlled trial supports the validity of multinational clinical trials.," J Endovasc The, vol. 21, no. 5, pp. 644-53. , 2014. |
|
[72] |
Siablis D1, et al., "Paclitaxel-coated balloon angioplasty versus drug-eluting stenting for the treatment of infrapopliteal long-segment arterial occlusive disease: the IDEAS randomized controlled trial.," JACC Cardiovasc Interv. 2014 Sep;7(9):, vol. 7, no. 9, pp. 1048-56., 2014. |
|
[73] |
Diamantopoulos A1, Gupta Y2, Zayed H3, Katsanos K3., "Paclitaxel-coated balloons and aneurysm formation in peripheral vessels," J Vasc Surg. 2015 Nov;62(5):, vol. 62, no. 5, pp. 1320-2., 2015. |
|
[74] |
Scheinert D1, et al, "The LEVANT I (Lutonix paclitaxel-coated balloon for the prevention of femoropopliteal restenosis) trial for femoropopliteal revascularization: first-in-human randomized trial of low-dose drug-coated balloon versus uncoated balloon angioplasty.," JACC Cardiovasc Interv. 2014 Jan;7(1):, vol. 7, no. 1, pp. 10-9., 2014. |
|
[75] |
Tepe G1, Zeller T, Schnorr B, Claussen CD, Beschorner U, Brechtel K, Scheller B, Speck U., "High-grade, non-flow-limiting dissections do not negatively impact long-term outcome after paclitaxel-coated balloon angioplasty: an additional analysis from the THUNDER study.," J Endovasc Ther., vol. 20, no. 6, pp. :792-800. , 2013. |
|
[76] |
Neizel M1, et al., "Monitoring of gadolinium-BOPTA uptake into the vessel wall during magnetic resonance (MR)-guided angioplasty of the peripheral arteries with a paclitaxel/gadolinium-BOPTA-coated balloon: an experimental study at 3 Tesla," Rofo. , vol. 186, no. 4, pp. 388-93., 2014. |
|
[77] |
Fusaro M1, Cassese S, Ndrepepa G, King LA, Tada T, Ott I, Kastrati A., "Paclitaxel-coated balloon or primary bare nitinol stent for revascularization of femoropopliteal artery: a meta-analysis of randomized trials versus uncoated balloon and an adjusted indirect comparison.," Int J Cardiol. 2013 Oct 9;168(4): ., vol. 168, no. 4, pp. 4002-9., 2013. |
|
[78] |
Schneider PA1, et al, "Mortality Not Correlated With Paclitaxel Exposure: An Independent Patient-Level Meta-Analysis of a Drug-Coated Balloon.," J Am Coll Cardiol., vol. 73, no. 20, pp. 2550-2563, 2019. |
|
[79] |
Buszman PP1,et al., "Experimental evaluation of pharmacokinetic profile and biological effect of a novel paclitaxel microcrystalline balloon coating in the iliofemoral territory of swine.," Catheter Cardiovasc Interv. , vol. 83, no. 2, pp. 325-33. , 2014. |
|
[80] |
Karimi A1, et al, "Randomized trial of Legflow(®) paclitaxel eluting balloon and stenting versus standard percutaneous transluminal angioplasty and stenting for the treatment of intermediate and long lesions of the superficial femoral artery (RAPID trial): study protocol fo," Trials. , 2013. |
|
[81] |
Bosiers M1, Peeters P, Tessarek J, Deloose K, Strickler S; Zilver PTX Single-Arm Study Investigators., "The Zilver® PTX® Single Arm Study: 12-month results from the TASC C/D lesion subgroup.," J Cardiovasc Surg (Torino)., vol. 54, no. 1, pp. 115-22, 2013. |
|
[82] |
Milewski K1, et al, "Evaluation of efficacy and dose response of different paclitaxel-coated balloon formulations in a novel swine model of iliofemoral in-stent restenosis.," JACC Cardiovasc Interv., vol. 5, no. 10, pp. 1081-8. , 2012 . |
|
[83] |
De Cock E1, et al., "A budget impact model for paclitaxel-eluting stent in femoropopliteal disease in France.," Cardiovasc Intervent Radiol. , vol. 30, no. 2, pp. 362-70, 2013. |
|
[84] |
Fischer D1, et al., "Paclitaxcel-coated balloon plus bare metal stent vs. sirolimus-eluting stent in de novo lesions: an IVUS study.," EuroIntervention. , vol. 8, no. 4, pp. 450-455, 2012. |
|
[85] |
Katsanos K1, et al "Risk of Death Following Application of Paclitaxel-Coated Balloons and Stents in the Femoropopliteal Artery of the Leg: A Systematic Review and Meta-Analysis of Randomized Controlled Trials.," J Am Heart Assoc. 2018, vol. 7, no. 24, 2018. |
|
[86] |
Krokidis M1, Spiliopoulos S, Katsanos K, Sabharwal T., "Peripheral applications of drug-coated balloons: past, present and future.," Cardiovasc Intervent Radiol. , vol. 36, no. 2, pp. 281-91., 2013. |
|
[87] |
Cassese S1, Byrne RA, Ott I, Ndrepepa G, Nerad M, Kastrati A, Fusaro M., "Paclitaxel-coated versus uncoated balloon angioplasty reduces target lesion revascularization in patients with femoropopliteal arterial disease: a meta-analysis of randomized trials.," Circ Cardiovasc Interv. 2012 Aug , vol. 5, no. 4, pp. 582-9., 2012. |
|
[88] |
Cioppa A1, et al P., "Combined treatment of heavy calcified femoro-popliteal lesions using directional atherectomy and a paclitaxel coated balloon: One-year single centre clinical results.," Cardiovasc Revasc Med., vol. 13, no. 5, pp. 219-23. , 2012. |
|
[89] |
Dake MD1, et al., "Paclitaxel-eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: twelve-month Zilver PTX randomized study results.," Circ Cardiovasc Interv. , vol. 5, no. 6, pp. 495-504., 2011. |
|
[90] |
Milewski K1, et al., "Paclitaxel-iopromide coated balloon followed by "bail-out" bare metal stent in porcine iliofemoral arteries: first report on biological effects in peripheral circulation.," EuroIntervention., vol. 7, no. 3, pp. 362-8., 2011. |
|
[91] |
Freyhardt P1, et al., "Plasma levels following application of paclitaxel-coated balloon catheters in patients with stenotic or occluded femoropopliteal arteries.," Rofo. 2011 May;183(5):, vol. 183, no. 5, pp. 448-55., 2011. |
|
[92] |
Higashitani M1, et al., "Efficacy of paclitaxel-eluting stent implantation in hemodialysis patients.," Heart Vessels. , vol. 26, no. 6, pp. 582-9., 2011. |
|
[93] |
Ansel GM1, Lumsden AB, "Evolving modalities for femoropopliteal interventions.," J Endovasc Ther. , pp. 82-97. , 2009. |
|
[94] |
Werk M1, et al., "Inhibition of restenosis in femoropopliteal arteries: paclitaxel-coated versus uncoated balloon: femoral paclitaxel randomized pilot trial.," Circulation. , vol. 118, no. 13, pp. 1158-65, 2008. |
|
[95] |
Kang HJ1, et al., "Effects of stem cell therapy with G-CSF on coronary artery after drug-eluting stent implantation in patients with acute myocardial infarction.," Heart. 2008 May;94(5), vol. 94, no. 5,., 2008. |
|
[96] |
Bracale UM1, et al"Treatment of de novo femoro-popliteal lesions with a new Drug Coated Balloon: early experience of a single Center in the first 50 patients.," Transl Med UniSa. , vol. 20, pp. 3-8, 2019. |
|
[97] |
Ding Y1, et al., "Comparison of Drug-Eluting Stent with Bare-Metal Stent Implantation in Femoropopliteal Artery Disease: A Systematic Review and Meta-Analysis.," Ann Vasc Surg. 2018 Jul;50:96-105. doi: 10.1016/j.avsg.2017.12.003. Epub 2018 Mar 4., vol. 50, pp. 96-105, 2018. |
|
[98] |
Micari A1, wt al; IN.PACT Global Study Investigators., "Drug-Coated Balloon Treatment of Femoropopliteal Lesions for Patients With Intermittent Claudication and Ischemic Rest Pain," JACC Cardiovasc Interv. , vol. 11, no. 10, pp. :945-953., 2018. |


