Drugs
Drugs
SFDA Obtains PIC/S Membership
2023-05-12
Saudi Food and Drug Authority (SFDA) joins Pharmaceutical Inspection Co-operation Scheme (PIC/S), which aims at harmonizing inspection procedures worldwide, facilitating cooperation and networking between pertinent authorities, and regional and international organizations. SFDA's acceded to PIC/S after having fulfilled all the requirements and standards required for membership and gained the approval of member states during the PIC/S meeting held in Geneva, Switzerland, on May 11-12, 2023. Saudi Arabia became the first Arab country to be admitted to PIC/S.
Other News
SFDA, HPRA Discuss Areas of Future Cooperation
2023-05-08
His Excellency the CEO of the Saudi Food and Drug Authority(SFDA), Dr. Hisham bin Saad Aljadhey, and his accompanying delegation met today, Monday, with the CEO of the Irish Health Products Regulatory Authority (HPRA), Dr. Lorraine Nolan.
This visit comes on the sidelines of SFDA official participation at the 4th meeting of the International Heads of Food Agencies Forum (IHFAF) taking place this year in Ireland.
Other News
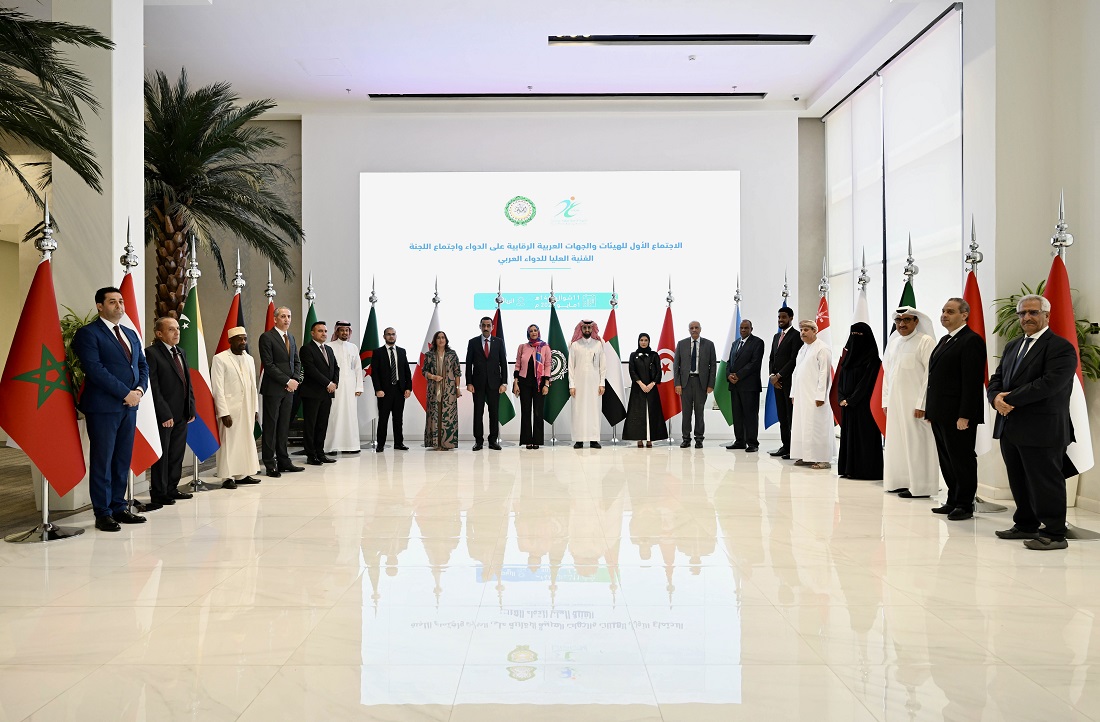
Saudi Arabia Hosts 1st Meeting of Arab Authorities Controlling Medicines
2023-05-01
The Kingdom of Saudi Arabia, represented by the Saudi Food and Drug Authority (SFDA), hosted the 1st Meeting of Arab Authorities Controlling Medicines today at the authority’s headquarters in Riyadh.
This meeting comes in implementation of Resolution No. (11) of the Council of Arab Ministers of Health in its (56th) regular session for 2022, as the Kingdom of Saudi Arabia welcomed at that time to host the aforementioned meeting, which is the first of its kind at the Arab level.
Other News
Specifications: Test Procedures and Acceptance Criteria for New Veterinary Drug Substances and New Medicinal Products: Chemical Substances (VICH GL39)
2023-03-29SFDA CEO inaugurates DIA Middle East Conference
2023-02-13
His Excellency the CEO of the Saudi Food and Drug Authority (SFDA), Dr. Hisham bin Saad Aljadhey, inaugurated the first Drug Information Association (DIA) Middle East Conference in Riyadh, which will be from 13 to 14 February 2023 in partnership with the SFDA.
Other News
- Previous page
- Page 15
- Next page