SFDA Organizes Two Specialized Workshops on Clinical Trials and Healthcare Technologies in the Medical Devices Sector at GHE
2025-10-30
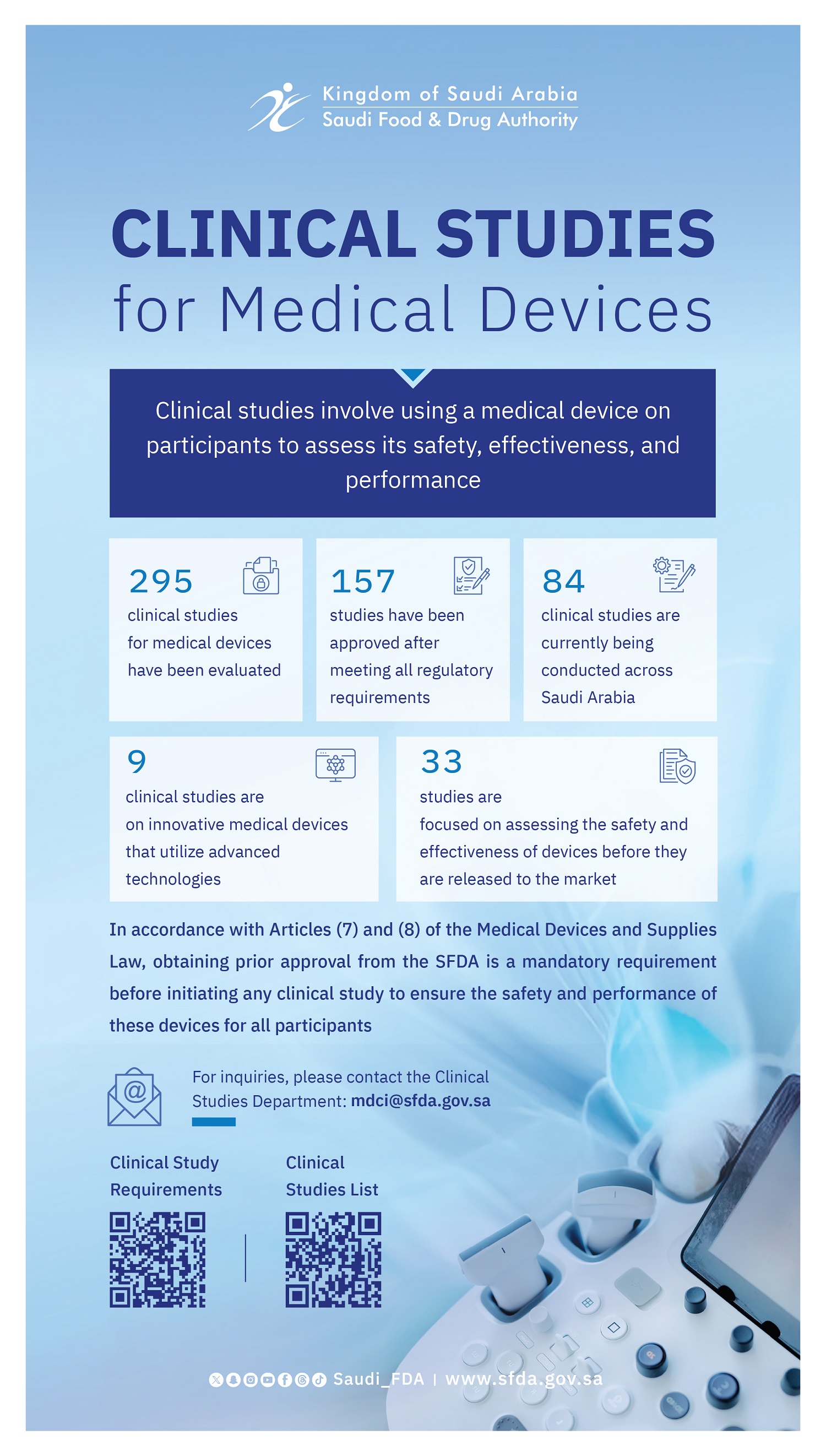
The Saudi Food and Drug Authority (SFDA) organized two specialized workshops on its Digital Health Platform as part of its participation in the Global Health Exhibition 2025, held in Riyadh under the theme “Invest in Health.” The workshops aimed to highlight leading regulatory practices in clinical trials for medical devices and in digital and emerging health technologies.