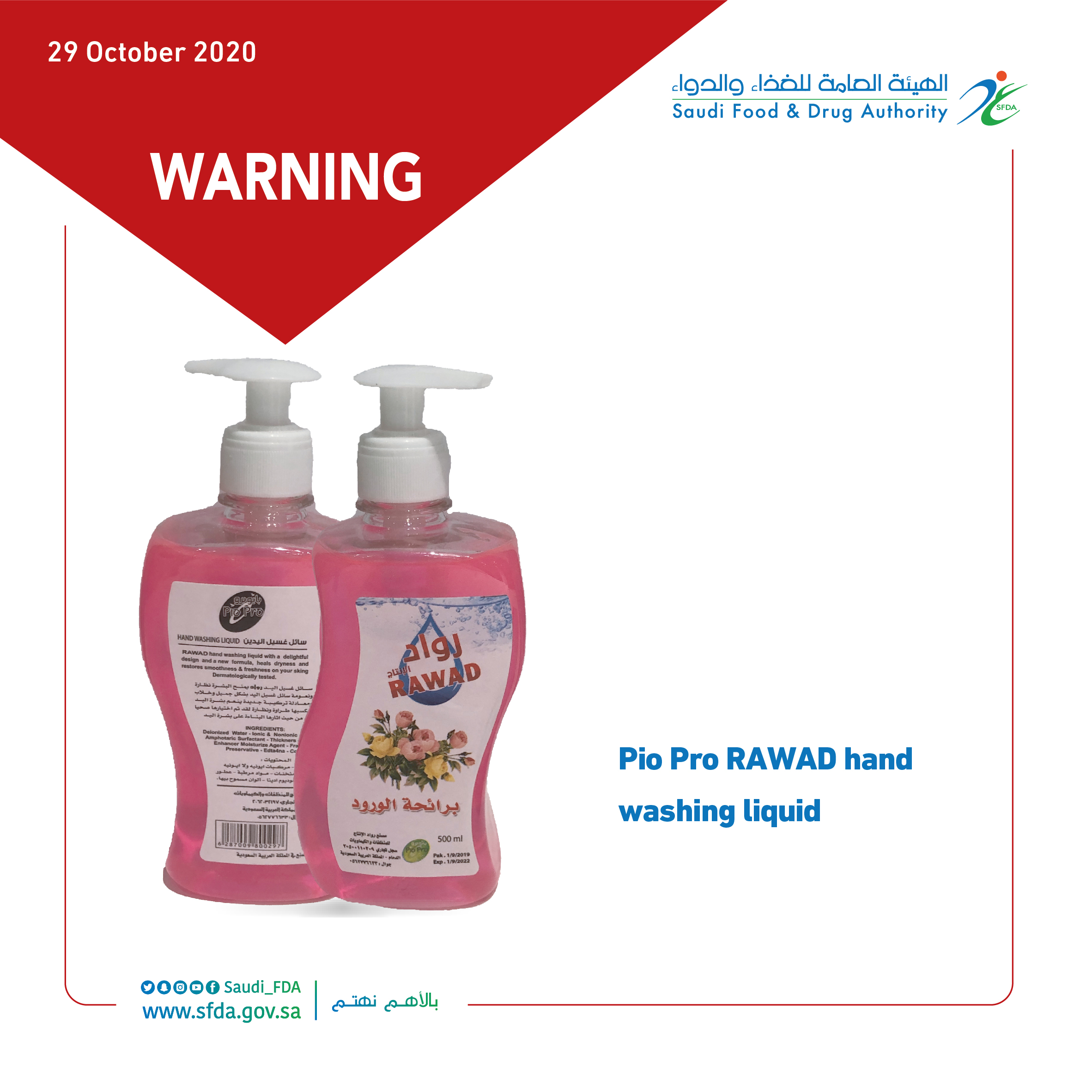
"SFDA" Warns Consumers against 13 Cosmetic Products Containing Elevated Levels of Sensitive Ingredients
2020-03-16
The Saudi Food and Drug Authority (SFDA) warned consumers not to use (13) cosmetic products that have elevated levels of sensitive ingredients.
The SFDA has explained that it collected and examined samples of cosmetic products from the local markets to ensure their safety.