Post-Market In-depth Clinical Evaluation for the Safety of Cap Disposable and Single-Use vs. Reusable Duodenoscopes for Endoscopic Retrograde Cholangiopancreatography
Post-Market In-depth Clinical Evaluation for the Safety of Cap Disposable and Single-Use vs. Reusable Duodenoscopes for Endoscopic Retrograde Cholangiopancreatography
Many infection outbreaks have been linked to contaminated duodenoscopes used for Endoscopic Retrograde Cholangiopancreatography (ERCP) procedures worldwide. Duodenoscopes are hollow, flexible, lighted tubes threaded through the mouth, throat, and stomach into the first part of the small intestine (duodenum). The complex design of duodenoscopes may impede effective reprocessing and lead to the transmission of the infection to patients who undergo ERCP with reprocessed duodenoscopes. The contributing factors of the duodenoscope design to contamination are not well understood. In 2013, the Centers for Disease Control and Prevention (CDC) notified the U.S. Food and Drug Administration (FDA) of a potential association between duodenoscopes and multi-drug resistant bacteria. The U.S. FDA has instigated huge investigations and recommendations for duodenoscopes.
The aim of this study is to evaluate the safety of cap disposable and single use duodenoscopes for Endoscopic Retrograde Cholangiopancreatography (ERCP) in terms of contamination compared with the standard types of duodenoscopes, which are reusable duodenoscopes.
Many infection outbreaks have been linked to contaminated duodenoscopes used for Endoscopic Retrograde Cholangiopancreatography (ERCP) procedures worldwide. Duodenoscopes are hollow, flexible, lighted tubes threaded through the mouth, throat, and stomach into the first part of the small intestine (duodenum). The complex design of duodenoscopes may impede effective reprocessing and lead to the transmission of the infection to patients who undergo ERCP with reprocessed duodenoscopes. The contributing factors of the duodenoscope design to contamination are not well understood. In 2013, the Centers for Disease Control and Prevention (CDC) notified the U.S. Food and Drug Administration (FDA) of a potential association between duodenoscopes and multi-drug resistant bacteria. The U.S. FDA has instigated huge investigations and recommendations for duodenoscopes.
The aim of this study is to evaluate the safety of cap disposable and single use duodenoscopes for Endoscopic Retrograde Cholangiopancreatography (ERCP) in terms of contamination compared with the standard types of duodenoscopes, which are reusable duodenoscopes.
Clinical need of Endoscopic retrograde cholangiopancreatography (ERCP):
Endoscopic retrograde cholangiopancreatography (ERCP) is a minimally invasive procedure and alternative to open surgery to diagnose and treat problems of the bile duct and pancreatic ducts. More than 650,000 patients undergo ERCP procedure in the US annually [1]. ERCP procedure combines X-ray and upper gastrointestinal (GI) endoscopy which is a long, flexible, lighted tube. Bile ducts are tubes which carry from the liver to gallbladder and duodenum, and pancreatic ducts are tubes which carry pancreatic juice from the pancreas to the duodenum (Figure 1). ERCP is performed using a duodenoscope. However, duodenoscope has complex design which make it difficult to clean properly. Insufficient cleaning of duodenoscope leads to patient-to- patient cross-contamination. Thus, contaminated duodenoscopes result in an increasing number of infectious. outbreaks involving multidrug-resistant organism [2].
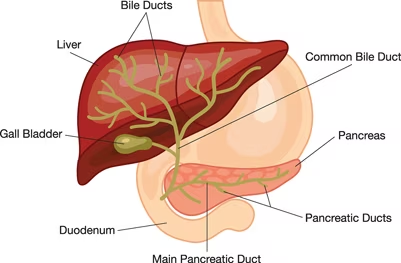
Figure1. the bile and pancreatic ducts[3]
Duodenoscopes:
In the US, duodenoscopes are used in more than 500,000 ERCP procedures annually. Duodenoscopes are hollow, flexible, lighted tubes threaded through the mouth, throat, and stomach into the first part of the small intestine (duodenum), and they are used for ERCP procedure. Reusable duodenoscopes are more complex than other endoscope devices that make them more difficult to clean and disinfect [4]. Duodenoscopes elevator channels can allow bacteria and microbes to remain after cleaning. Figure 2 shows the main parts of the duodenoscope[5]. There are other types of duodenoscopes which are the cap disposable duodenoscopes and single-use duodenoscope. They have been recently developed to decrease risk of infection transmission from contaminated reusable duodenoscopes. However, cap disposable duodenoscopes can reduce the risk of contamination but not eliminate it[6].
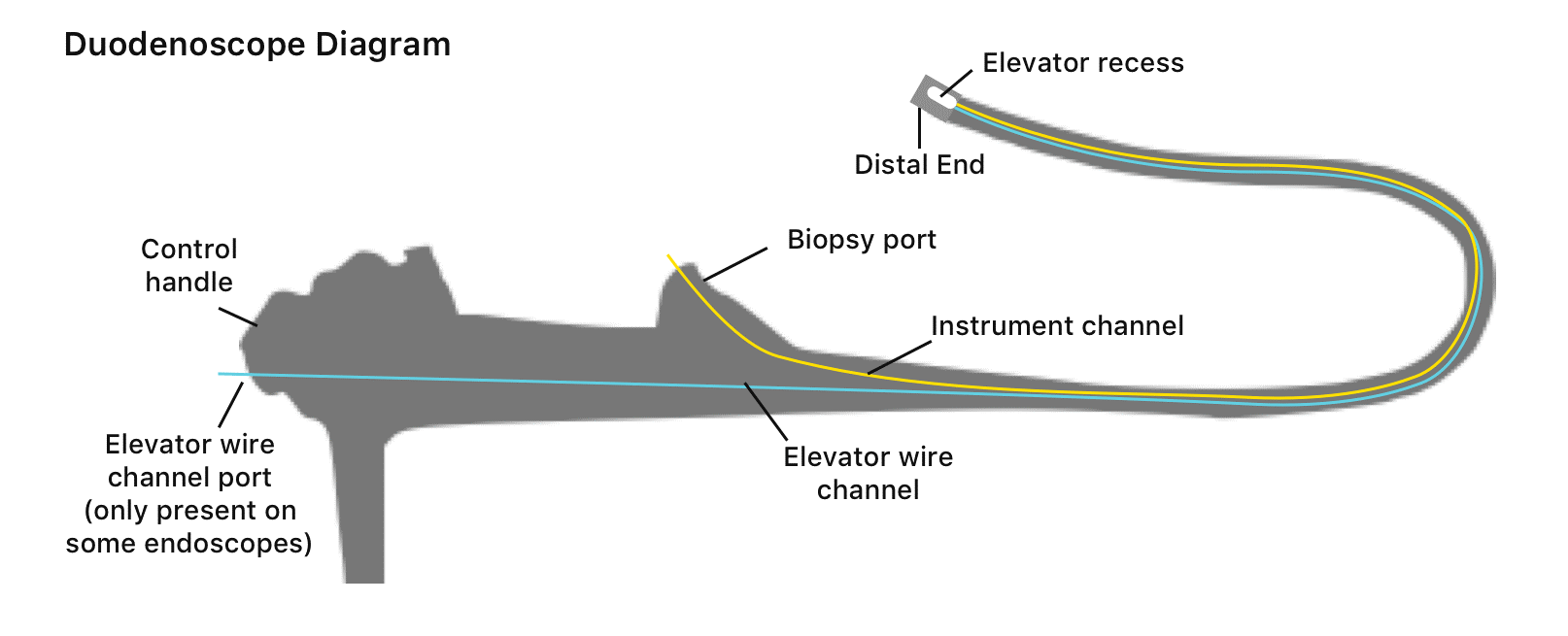
Figure2. The main parts of the duodenoscopes
Part 1: Clinical paper review
-
- An overview of search criteria
A systematic review was conducted to evaluate the safety of cap disposable and single use duodenoscopes for Endoscopic Retrograde Cholangiopancreatography (ERCP) in terms of contamination compared with the standard types of duodenoscopes, which are reusable duodenoscopes. Using a defined inclusion criterion, a total of 71 article were obtained and screened, which result in 12 articles, as shown in figure 3.
Figure 3. PRISMA flowchart for electronic database
2.1 Results of the clinical paper review
A number of 12 studies were included in this evaluation to assess the safety of cap disposable duodenoscopes and single-use duodenoscopes devices for Endoscopic Retrograde Cholangiopancreatography (ERCP) and compare them with reusable duodenoscopes. Table 2 compares the contamination rate between the standard model of duodenoscopes (reusable) and the cap disposable of duodenoscopes. As shown in Table 1, studies indicated that the cap disposable duodenoscopes reduced the contamination rate compared with standard duodenoscopes without affecting the technical performance and safety of ERCP. As a result, the cap disposable duodenoscope could be a novel technique that can help to lower the contamination rate after reprocessing and decrease healthcare-associated infections related to ERCP procedures but not eliminate them. In addition, there are some factors that can help to reduce the contamination rate of the reusable duodenoscopes, such as additional improvements to the IFU of the manufacturer, training programs for reprocessing procedure, applying a robust system of microbiological surveillance, cleanliness of reprocessing environments, and a distal end of the duodenoscope design. All these factors should be considered. However, the relationship between the duodenoscope and the contamination is unclear, and the evidence is insufficient to prove that. Because of that, further studies should address issues with contaminated reusable duodenoscopes that lead to cross-infections and patient harm following ERCP by reusable duodenoscopes.
In addition, Table 2 compares the performance of the single use duodenoscopes with the standard model of duodenoscopes (reusable), the results indicated that single use duodenoscopes are an effective alternative method to reusable duodenoscopes for performing ERCP procedures by expert endoscopists. The overall safety profile and technical performance are similar in both types of duodenoscopes. Moreover, single use duodenoscopes reduce and eliminate the risk of related infection.
Table 2: Clinical paper review for the safety of standard duodenoscopes (reusable) and cap disposable duodenoscopes:
|
Ref |
Study design |
Year |
Sample Size |
Contamination rate |
Comments |
||
|
#The standard model of duodenoscopes (Reusable) |
#The cap disposable duodenoscopes |
The standard model of Duodenoscopes |
The cap disposable duodenoscopes |
||||
|
]7[ |
Randomized Clinical Trial |
2023 |
259 samples of standard duodenoscopes
|
259 samples of Disposable elevator cap duodenoscopes |
11.2%
|
3.8% |
In this study, disposable elevator cap duodenoscopes reduced the contamination rate compared with standard duodenoscopes, without affecting the technical performance and safety of ERCP. |
|
]8[
|
Randomized Clinical Trial |
2022 |
200 samples of standard duodenoscopes |
200 samples of disposable distal caps duodenoscope,
|
14%
|
7%
|
In this study, duodenoscopes with disposable distal caps had significantly lower the contamination rate. |
|
]9[
|
Prospective surveillance trial
|
2022 |
16 samples of standard duodenoscopes
|
92 samples of disposable elevator cap duodenoscopes |
81.3% |
23.9% |
The study showed that disposable elevator cap duodenoscopes significantly reduced the rate of contamination after reprocessing. It is a novel design and cost-effective that can decrease healthcare associated infections (HAI) related to ERCP procedures. |
|
]10[ |
Prospective study |
2022 |
N/A |
46 samples of disposable tips dudenoscope |
N/A |
Low concern organisms were 2.7%
High concern organisms were 0% |
This study compared to FDA data from reusable Pentax duodenoscopes, and it was found that the rates of low/moderate-concern organism contamination and high-concern organism contamination of duodenoscopes with disposable tips were lower. As a result, the disposable tips decrease bacterial contaminations but do not eliminate them. |
|
]11[
|
A systematic review and meta-analysis
|
2020 |
2,560 samples of standard duodenoscopes |
N/A |
15.34% |
N/A |
The study showed that the contamination rate was 15.34%. The study indicated that more studies should be conducted to address issues with contaminated reusable duodenoscopes that leads to cross-infections and patient harm following ERCP by reusable duodenoscope. |
|
]12[
|
Descriptive study |
2020 |
350 samples of standard duodenoscopes |
N/A |
26.5% |
N/A |
The study showed the risk of contamination through the use of duodenoscope and the importance of applying robust system of microbiological surveillance and conducting training programs, resulting in decrease the contamination rate and the risk of spreading retrograde cholangiopancreatography (ERCP)-associated infections. |
|
]13[
|
A systematic review and meta-analysis |
2020 |
13,112 samples of standard duodenoscopes |
N/A |
15.25% |
N/A |
The study showed that the contamination rate was 15.25%. Moreover, the study indicated that the present reprocessing methods are not effective and proper regards to cleaning duodenoscopes. Further studies should be conducted to address issues with contaminated reusable duodenoscopes that leads to cross-infections and patient harm following ERCP by reusable duodenoscope. |
|
]14[
|
Cross-sectional study |
2017 |
69 samples of standard duodenoscopes
43 samples of standard duodenoscopes
|
N/A |
22%
30% |
N/A |
This study shows high rates of the contamination of reusable duodenoscopes for ERCP. These results concluded that the current reprocessing procedures are not safe and sufficient. |
Table 3: Clinical paper review for the safety of standard duodenoscopes (reusable) and the single use of duodenoscopes:
|
Study design |
Year |
Sample Size |
Findings of the study |
Conclusion of the study |
||
|
#The standard model of Duodenoscopes (Reusable) |
||||||
|
]15[
|
Randomized Clinical Trial |
2021 |
50 patients who underwent standard duodenoscopes
|
48 patients who underwent Single-use duodenoscopes.
|
|
Single-use duodenoscopes are an alternative to reusable duodenoscope for performing ERCP procedures in skilled hands. The overall safety profile and technical performance are similar in both types of duodenoscopes. |
|
]16[
|
Case-series study |
2019 |
N/A |
73 patients who underwent Single-use duodenoscopes.
|
|
ERCPs can be completed successfully of a wide range of complexity using a single-use duodenoscope by expert endoscopists for almost all cases. Single-use duodenoscopes are alternative to reusable duodenoscope, and they might reduce ERCP-related risk of infection. |
|
]17[
|
Retrospective study |
2021 |
N/A |
32 patients who underwent Single-use duodenoscopes
|
|
Single-use duodenoscopes eliminate the risk of related infection, and they are effective alternative methods to standard reusable duodenoscopes. |
Part 2: Clinical experience review
2.1 International regulatory organizations opinions
U.S. Food and Drug Administration (FDA)
In 2013, the Centers for Disease Control and Prevention (CDC) notified the Food and Drug Administration (FDA) of a potential association between duodenoscopes and multi-drug resistant bacteria. The FDA has instigated huge investigations and recommendations for duodenoscopes since 2013. These include post-marketing surveillance studies, manufacturer guidance changes, and recommendations to use cap disposable duodenoscopes or fully disposable duodenoscope to lower risks of infection.
Saudi food and Drug Authority (SFDA):
In 2022, SFDA conducted a risk analysis report by the risk analysis department in the medical devices sector to investigate and analyze the infected duodenoscopes. The result showed several precautions and warnings in the reprocessing manual, and other important information needs to be considered before and after reprocessing the duodenoscope. The study recommended that a reprocessing person must have sufficient training, follow the instructions of reprocessing manuals, continue following up with the duodenoscope in terms of adverse events report, and encourage healthcare providers to report adverse events.
In addition, SFDA released a safety communication about the potential risk of infections associated with reprocessed duodenoscopes to all healthcare providers. The recommendations include developing a process to ensure that reprocessing employees follow the manufacturer's IFU and take appropriate action when deviations occur before acquiring a new duodenoscope to ensure the reprocessing capabilities of the healthcare facility are compatible with the duodenoscope technology.
2.2 The opinion of Saudi experts and users:
Saudi healthcare providers
A survey about the safety of cap disposable and single use duodenoscopes devices was prepared and sent to the targeted healthcare providers (N = 40) around Kingdom of Saudi Arabia (KSA). The result showed that reusable duodenoscopes and cap disposable duodenoscopes are most common to use in the KSA. Moreover, 86% of healthcare providers conduct routine inspection and periodic maintenance in accordance with the manufacturer's instructions for duodenoscope, and 64% of healthcare providers have a quality control program. On the other hand, they conduct reprocessing duodenoscope with supplemental measures, so the healthcare providers are compliance with reprocessing procedure for duodenoscope. Finally, 79% of healthcare providers believe that cap disposable duodenoscope or fully disposable duodenoscope (single use) reduce contamination rate and risk of infection.
Part 3: Overall conclusion
To sum up, cap disposable duodenoscope and single-use duodenoscopes decrease the contamination rate and risk of infection compared with reusable duodenoscopes. In addition, some factors could help reduce the contamination rate and risk of infection of reusable duodenoscopes, which are appropriate reprocessing procedures, trained staff who conduct the reprocessing procedure, and following the manufacturer instructions for reprocessing procedures.
Thanks to the post-market clinical evaluation team for their supports in conducting this work.
For further information or inquiries related to this study, you may contact us at: cia.md@sfda.gov.sa
- Okamoto N, Sczaniecka A, Hirano M, et al (2022) A prospective, multicenter, clinical study of duodenoscope contamination after reprocessing. Infect Control Hosp Epidemiol 43:1901–1909
- Endoscopic Retrograde Cholangiopancreatography (ERCP) - NIDDK. https://www.niddk.nih.gov/health-information/diagnostic-tests/endoscopic-retrograde-cholangiopancreatography. Accessed 12 Aug 2023
- Endoscopic Retrograde Cholangiopancreatography (ERCP) - NIDDK. https://www.niddk.nih.gov/health-information/diagnostic-tests/endoscopic-retrograde-cholangiopancreatography. Accessed 12 Aug 2023
- Health C for D and R (2023) Infections Associated with Reprocessed Duodenoscopes. FDA
- Duodenoscope: What Is a Duodenoscope & How Does It Work? In: Drugwatch.com. https://www.drugwatch.com/duodenoscope/. Accessed 12 Aug 2023
- Health C for D and R (2022) Use Duodenoscopes with Innovative Designs to Enhance Safety: FDA Safety Communication. FDA
- Forbes N, Elmunzer BJ, Allain T, et al (2023) Effect of Disposable Elevator Cap Duodenoscopes on Persistent Microbial Contamination and Technical Performance of Endoscopic Retrograde Cholangiopancreatography. JAMA Intern Med 183:191–200Sanders DJ, Bomman S, Krishnamoorthi R, Kozarek RA (2021) Endoscopic retrograde cholangiopancreatography: Current practice and future research. World J Gastrointest Endosc 13:260–274
- Ridtitid W, Thummongkol T, Chatsuwan T, Piyachaturawat P, Kulpatcharapong S, Angsuwatcharakon P, Mekaroonkamol P, Kongkam P, Rerknimitr R (2022) Bacterial contamination and organic residue after reprocessing in duodenoscopes with disposable distal caps compared with duodenoscopes with fixed distal caps: a randomized trial. Gastrointest Endosc 96:814–821
- Bhakta D, Joseph-Talreja M, DaVee T, Wadhwa V, Ramireddy S, Rashtak S, Guha S, Patil P, Ostrosky L, Thosani N (2022) sterile disposable elevator cap (dec) significantly reduces rate of persistent duodenoscope contamination after reprocessing: outcomes from a prospective surveillance trial. gastrointestinal endoscopy 95:ab33
- Muralidharan S, Parish A, Niedzwiecki D, et al (2022) S1071 Assessment of Pathogenic Enteric Flora Contaminating Novel Duodenoscopes With Disposable Tips. Official journal of the American College of Gastroenterology | ACG 117:e776
- Larsen S, Russell RV, Mærkedahl A, Travis HS, Ockert LK, Ehlers LH (2020) Contamination rate of reusable patient-ready duodenoscopes used for endoscopic retrograde cholangio-pancreatography (ercp): Digestive Disease Week 2020. Gastroenterology 158:S-1004-S-1005
- Cristina ML, Sartini M, Schinca E, Ottria G, Dupont C, Bova P, Coccia G, Casini B, Spagnolo AM (2020) Is Post-Reprocessing Microbiological Surveillance of Duodenoscopes Effective in Reducing the Potential Risk in Transmitting Pathogens? Int J Environ Res Public Health 17:140
- Larsen S, Russell RV, Ockert LK, Spanos S, Travis HS, Ehlers LH, Mærkedahl A (2020) Rate and impact of duodenoscope contamination: A systematic review and meta-analysis. EClinicalMedicine 25:100451
- Rauwers AW, Voor In ’t Holt AF, Buijs JG, de Groot W, Hansen BE, Bruno MJ, Vos MC (2018) High prevalence rate of digestive tract bacteria in duodenoscopes: a nationwide study. Gut 67:1637–1645
- Equivalent performance of single-use and reusable duodenoscopes in a randomised trial | Gut. https://gut.bmj.com/content/70/5/838. Accessed 12 Aug 2023
- Muthusamy VR, Bruno MJ, Kozarek RA, et al (2020) Clinical Evaluation of a Single-Use Duodenoscope for Endoscopic Retrograde Cholangiopancreatography. Clin Gastroenterol Hepatol 18:2108-2117.e3
Ehrlich D, Chittajallu P, Phan J, Paredes HE, Issa D, Thaker AM, Kim S, Sedarat A, Muthusamy VR (2021) ID: 3526115 the exalt model d single-use duodenoscope is safe and effective in post-market experience at a tertiary medical center. gastrointestinal endoscopy 93:ab171


