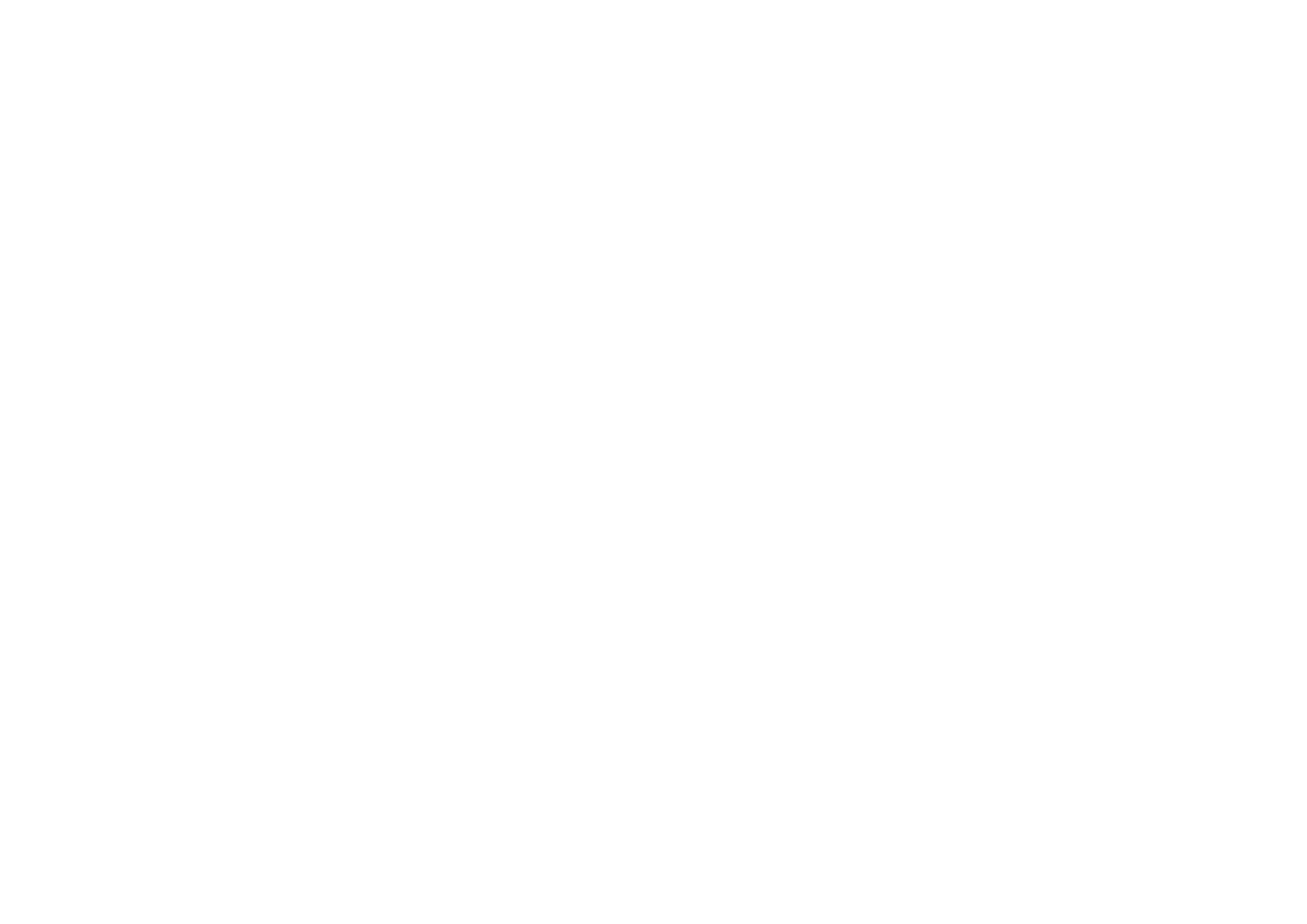
Periodic safety reporting schedule
Periodic safety reporting schedule
| Active Substances | Trade Name | MAH | Year of registration in Saudi Arabia | International Birth Date (IBD) | PSUR Submission Frequency | Data Lock Point (DLP) | Submission Date |
|---|---|---|---|---|---|---|---|
| Dulaglutide | TRULICITY |
Eli Lilly | 2017 | 2014-09-18 | 6 months | 2017-09-18 | 2017-11-27 |
| Rivaroxaban | XARELTO |
BAYER PHARMA AG | 2011 | 2008-09-15 | annual | 2017-09-15 | 2017-11-24 |
| Leflunomide | RHEUFACT |
Jazeera Pharmaceutical Industries (JPI) | 2012 | 1998-09-10 | annual | 2017-09-10 | 2017-11-19 |
| Naproxen | NAPROX |
Hikma Pharmaceuticals PLC | 1997 | 1961-04-08 | 3 years | 2017-08-03 | 2017-11-01 |
| Duloxetine | Cymbalta |
Eli Lilly | 2010 | 2004-08-03 | 3 years | 2017-08-03 | 2017-11-01 |
| Duloxetine | Cymbalta |
Eli Lilly | 2006 | 2004-08-03 | 3 years | 2017-08-03 | 2017-11-01 |
| Naproxen | Aleve |
BAYER PHARMA AG | 2017 | 1975-11-07 | 3 years | 2017-08-03 | 2017-11-01 |
| Duloxetine | DELAXIN |
Jazeera Pharmaceutical Industries (JPI) | 2016 | 2004-08-11 | 3 years | 2017-08-03 | 2017-11-01 |
| Fenofibrate | LIPANTHYL |
ABBOTT Laboratories | 1998 | 1974-04-11 | 3 years | 2017-07-31 | 2017-10-29 |
| Quetiapine | QUETAL |
Jazeera Pharmaceutical Industries (JPI) | 2015 | 1997-07-31 | 3 years | 2017-07-31 | 2017-10-29 |